Journal:Potency and safety analysis of hemp-derived delta-9 products: The hemp vs. cannabis demarcation problem
| Full article title | Potency and safety analysis of hemp delta-9 products: The hemp vs. cannabis demarcation problem |
|---|---|
| Journal | Journal of Cannabis Research |
| Author(s) | Johnson, Lee; Malone, Marc; Paulson, Erik; Swider, Josh; Marelius, David; Andersen, Susan; Black, Dominic |
| Author affiliation(s) | CBD Oracle, Infinite Chemical Analysis Labs |
| Primary contact | Email: lee at cbdoracle dot com |
| Year published | 2023 |
| Volume and issue | 5 |
| Article # | 29 |
| DOI | 10.1186/s42238-023-00197-6 |
| ISSN | 2522-5782 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://jcannabisresearch.biomedcentral.com/articles/10.1186/s42238-023-00197-6 |
| Download | https://jcannabisresearch.biomedcentral.com/counter/pdf/10.1186/s42238-023-00197-6.pdf (PDF) |
Abstract
Background: Hemp-derived delta-9-tetrahydrocannabinol (Δ9-THC) products are freely available for sale across much of the USA, but the federal legislation allowing their sale places only minimal requirements on companies. Products must contain no more than 0.3% Δ9-THC by dry weight, but no limit is placed on overall dosage, and there is no requirement that products derived from hemp-based Δ9-THC be tested. However, some states—such as Colorado—specifically prohibit products created by “chemically modifying” a natural hemp component.
Methods: Fifty-three hemp-based Δ9-THC products were ordered and submitted to InfiniteCAL laboratory for analysis. The lab analysis considered potency, the presence of impurities, and whether the Δ9-THC present was natural or converted from cannabidiol (CBD). The presence of age verification, company-conducted testing, and warning labels was also considered.
Results: While 96.2% of products were under the legal Δ9-THC limit, 66.0% differed from their stated dosage by more than 10%, and although 84.9% provided a lab report to customers, 71.1% of these did not check for impurities. Additionally, 49% of products converted CBD to THC to achieve their levels, and only 15.1% performed age verification at checkout.
Conclusions: Despite some positive findings, the results show that hemp-derived Δ9-THC companies offer inaccurately labeled products that contain more THC than would be allowed in adult-use states. This raises serious issues around consumer safety, and consent when consuming intoxicating products. Steps to boost accountability for companies must be considered by either the industry or lawmakers if intoxicating hemp products are to safely remain on the market.
Keywords: hemp, Δ9-THC, Farm Bill, Agriculture Improvement Act, cannabinoid potency
Background
Delta-9-tetrahydrocannabinol (Δ9-THC) is the primary psychoactive component of the Cannabis sativa L. plant [Cooper and Haney 2009], with other cannabinoids like cannabidiol (CBD) attracting attention for their therapeutic properties [Russo and McPartland 2003] in recent years. [National Academies of Sciences, Engineering, and Medicine (NASEM), 2017] While both cannabinoids have medical applications, Δ9-THC has largely been associated with recreational use. Until 2012 [Conference and of State Legislatures (NCSL): State Medical Cannabis Laws 2022], the prohibition of the recreational use of cannabis in the United States has made it essentially impossible to obtain legally, except through certain medical channels.
However, things changed when the Agriculture Improvement Act of 2018 (a.k.a. the “Farm Bill”) made industrial hemp legal at the federal level. [Agriculture Improvement Act of (US), 2018] The legislation allowed for an explosion of CBD products, but there were unintended consequences. The Farm Bill removed the cannabinoids in hemp from the definition of "marijuana" in the Controlled Substances Act and defined hemp as containing less than 0.3% Δ9-THC by dry weight. [Johnson-Arbor and Smolinske 2022] This allowed non-intoxicating CBD oils, for example, to be sold freely. However, loopholes quickly emerged, such as ignoring the matter of Δ8-THC, another psychoactive compound much like Δ9-THC except with less potent and long-lasting effects [Kruger et al. 2022b] and less binding affinity for the CB1 receptor. [Tagen and Klumpers 2022] Since it is a natural component of hemp, provided that products containing it have less than 0.3% Δ9-THC by dry weight, they can contain as much Δ8-THC as they want. Some states have taken action to stop the sale and distribution of Δ8-THC [Johnson-Arbor and Smolinske 2022], but new loopholes (for example, the increase in products with hexahydrocannabinol [HHC] [Casati et al. 2022]) are identified more quickly than lawmakers can close them.
While Δ8-THC is present in negligible amounts in the Cannabis plant, virtually all products sold to consumers use Δ8-THC produced from CBD (Tagen and Klumpers 2022) by cyclization (the closure of a ring after an acid-catalyzed activation of a double bond). [Marzullo et al. 2020] This creates potential legal issues at the federal level (because it may render it “synthetic” THC), but the conversion process itself has also been a target of state-level legislation. [CO Department of Public Health and Environment (DPHE): Re: Production and/or Use of Chemically Modified or Converted Industrial Hemp Cannabinoids 2021; Commonwealth of Massachusetts: Hemp in Massachusetts: Faqs 2022; SB 0788 (Md.) 2022]
Hemp-derived Δ9-THC products were devised through a very simple application of the Farm Bill’s 0.3% by dry weight limit. A 10 g gummy can contain roughly 10 g × 0.3% = 0.03 g = 30 mg of Δ9-THC and still be within the legal limit. In contrast, intoxicating cannabis edibles in legal states like California and Colorado tend to contain just 5 mg or 10 mg Δ9-THC per serving. [Brangham 2014; Romine 2019] As an unavoidable consequence of the law as it is currently written, intoxicating “hemp” Δ9-THC products are widely available in most states.
There are many potential issues with this; however, the biggest is the minimal regulations imposed on these “hemp” companies, especially in comparison to the regulations of legal cannabis markets. For instance, in California [Medicinal and Adult-Use Commercial CannabisRegulations (CA) 2023], each product must be lab tested for cannabinoid potency, residual pesticides, foreign material, heavy metals, microbial impurities, mycotoxins, moisture content, and residual solvents, and packaging must be child-resistant, tamper-evident, and resealable, containing a cannabis universal symbol and numerous other pieces of information, such as a batch number and a full ingredient listing. These and similar regulations protect consumers in states with legal cannabis, but are not a requirement for hemp under the Farm Bill.
Since hemp-derived Δ9-THC products are intoxicating, many people argue that they should meet similar standards to edibles in states like California and Colorado [Hemp and Roundtable: Delta-8 2021], and be subject to the same requirements for things like warning labels and child-safe packaging. As with Δ8-THC products, it is also possible that some of the Δ9-THC in hemp products is created through cyclization, and consequently may be impacted by existing state legislation.
This study aims to investigate the hemp-derived Δ9-THC market with this in mind. In particular, we aim to determine whether companies remain within legal limits, whether the stated dosages are accurate, whether the Δ9-THC was produced by cyclization, and whether companies performed safety testing on products and made sufficient effort to prevent minors from purchasing them.
Methods
Sample size and product selection
For the lab study and market analysis, we first identified and purchased a selection of the most popular products online from different brands. To identify brands, Google searches for “hemp delta-9 thc edibles,” “hemp delta-9 thc tinctures,” “hemp delta-9 thc vapes,” “hemp delta-9 thc products,” “full spectrum CBD + THC product,” and “compliant delta-9 thc product” were performed, and the first 20 pages of results (200 search results total) were reviewed. The relevant commercial results were selected, excluding third-party lists of products and educational content. In the event this process led to a specific product, we navigated to the overall category page for hemp-derived Δ9-THC products. We also searched Reddit, Instagram, and YouTube to identify brands that were missed by the Google search. Companies listed on CBD Oracle’s internal database of hemp companies were also manually searched for hemp-derived Δ9-THC products. In total, we identified 89 brands currently selling hemp-derived Δ9-THC products.
We estimated that this represents around 75% of the total hemp-derived Δ9-THC market, as of April 2022. It is unlikely that the search strategy identified all brands, particularly local brands or those with no online presence. It was estimated, as a result of this and our overall familiarity with the market, that the strategy captured around 75% of the market. This estimate is imperfect by definition, because it cannot be precisely known how many brands exist beyond the boundaries of our online search. With this in mind, we estimate that there were 120 brands selling hemp-derived Δ9-THC products as of April 2022.
To select specific products for the lab analysis, companies with a TrustPilot rating lower than "4" or with a Better Business Bureau (BBB) grade below "B" were excluded, as were any companies which didn’t ship to California and any products that didn’t mention a specific dosage of THC. Companies were also ranked for popularity using the number of customer reviews on-site and followings on social media websites. We selected the Δ9-THC product from each company with the highest number of customer reviews. In some cases, we bought multiple products from the same manufacturer to cover more types of product.
We ordered a total of 53 products with a credit card and had them shipped to the CBD Oracle office in Tustin, CA. However, owing to the nature of the market, the majority of them were edibles. The products included gummies (38 products), tinctures (3), vape pens (1), cookies (2), brownies (1), chocolate (1), candies (3), beverages (3), and rice krispies (1). The vast majority sold some form of edibles (total 46), but we identified a few companies offering tinctures, some offering beverages, and one that offers a vape pen. The 53 products came from 48 different brands, which we estimate represented 40% of the total hemp-derived Δ9-THC product market, as of April 2022. Manufacturers came from multiple states: AZ, CA, CO, FL, GA, IN, MA, MI, MN, MO, NC, NJ, NV, NY, OR, TN, TX, and WI.
Lab analysis
Products were collected for lab analysis directly from the office in their original, sealed packaging and were tested within two weeks of purchase to avoid degradation. The lab analyses were performed by InfiniteCAL, a California Department of Cannabis Control (DCC)- and International Organization for Standardization/International Electrotechnical Commission (ISO/IEC 17025)-accredited lab. All products were tested for potency, and 10 randomly selected products were tested for impurities, including pesticides, mycotoxins, residual solvents, microbial contamination, heavy metals, and foreign materials.
Potency analyses for the mass/mass percentage concentrations of cannabinoids (Δ9-THC, Δ8-THC, CBD, tetrahydrocannabinolic acid [THCA], cannabidiolic acid [CBDA], cannabigerol [CBG], cannabigerolic acid [CBGA], cannabinol [CBN], tetrahydrocannabivarin [THCV], cannabidivarin [CBDV], and cannabichromene [CBC]) were performed using ultra-high-performance liquid chromatography coupled with a diode array detector (UHPLC-DAD), and concentrations are determined in relation to a calibration curve established based on certified reference materials.
Δ9-THC was extracted from the gummies/edibles using one of two parallel validated procedures. The standard procedure is as follows: Solid edible samples are cryoground to a fine powder to ensure homogeneity. A subsample (3.0 g) is weighed in a 50-mL centrifuge tube containing steel balls. Forty milliliters of methanol is added to the tube and subsequently weighed to determine the exact volume of solution. Solutions are then sonicated in a 55 °C water bath then vortexed. Solid edibles should be reduced to a silt consistency, and therefore, it may be necessary to repeat and alternate sonication and vortexing steps. After the desired consistency is reached, samples are centrifuged for three minutes at 4200 rpm. A subsequent dilution step is performed with methanol in a separate 15-mL tube. Solutions are then filtered with a 0.22-um filter into a 2-mL autosampler vial.
For samples suspected or confirmed to contain gelatin (which includes samples that do not reach the desired consistency using the standard procedure), an alternate similar procedure mirrors the standard preparation with the following changes: a mixture of 50:50 water/methanol is used in place of methanol for the extraction step, and the dilution step uses acetonitrile instead of methanol and is also centrifuged for three minutes at 4200 rpm.
The measured potency depends on how well the THC was extracted from the products. However, InfiniteCAL has performed extensive validation on edible products, with both internal sample recovery experiments (using edibles spiked with known amounts of cannabinoids) as well as external blind proficiency tests, which have shown the extraction and analysis techniques used to be both thorough and robust. Validation data can be provided upon request.
Pesticide and mycotoxin levels were determined using a combination of gas chromatography triple quad mass spectrometry (GC MS/MS) and liquid chromatography triple quad mass spectrometry (LC-MS/MS). Concentrations of arsenic (As), cadmium (Cd), mercury (Hg), and lead (Pb) were determined using inductively coupled plasma mass spectrometry (ICP-MS). Analyses for heavy metals were conducted in kinetic energy discrimination (KED) mode, using helium (He) as the collision gas and argon (Ar) as the carrier gas.
Residual solvent and terpene analyses were performed using headspace gas chromatography single quad mass spectrometry (HS-GC-MS). Microbial analysis was performed using real-time polymerase chain reaction (qPCR), with aliquots taken from the batch being incubated for 24 hours to allow microbial growth before testing. Moisture content was determined using a moisture analyzer, with loss of moisture from a pre-defined sample calculated gravimetrically. Finally, foreign material testing was performed visually, either unaided or with a microscope magnifier.
Full details of the methodology are available from InfiniteCAL. [Swider and Marelius 2022]
Δ9-THC conversion markers
Samples were analyzed to determine whether the Δ9-THC present was naturally occurring in the hemp plant or whether it was produced through conversion from CBD. While it is not possible to determine the source of the Δ9-THC with absolute certainty, there are several indicators that strongly suggest one of three sources: the Δ9-THC naturally produced by a hemp plant, Δ9-THC sourced from a cannabis plant, and Δ9-THC resulting from a conversion from CBD. Additional File 1 contains more detail about the markers used and the underlying chemistry. Note that these analyses were only performed with 49/53 products, due to very low quantities of minor cannabinoids in three samples, which made identification of the source challenging, and the remaining sample contained no THC.
Trans:cis-Δ9-THC ratio
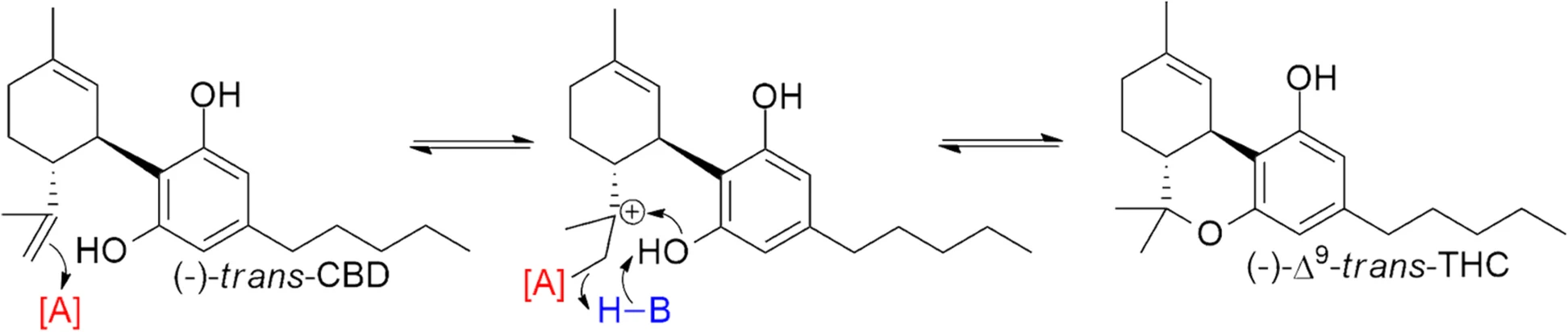
Schafroth et al. [Schafroth et al. 2021] found that while cis-Δ9-THC was entirely absent from a high-THC Bedrocan cultivar, 28/31 (90.3%) of hemp plants had trans:cis ratios between 1.3:1 and 8:1. These observations suggest that the biosynthetic pathways to produce Δ9-THC in classical hemp strains are not stereospecific and produce both trans-Δ9- and cis-Δ9-THC, while high-THC cannabis strains have a strongly stereospecific pathway to produce the (-)-trans-Δ9-THC. The delineation of the two strain types based on the presence/absence of cis-Δ9-THC can therefore provide potential markers to identify the source of Δ9-THC.
Since it is possible to synthetically form (-)-trans-Δ9-THC directly from (-)-trans-CBD through an isomerization-free mechanism (Fig. 1), the ratios of trans:cis-Δ9-THC in distillate converted from CBD can be expected to far exceed the ratios seen in natural hemp extracts. In contrast, oxidative cyclization from CBGA is the main source for biosynthesized THCA [Taura et al. 2007], and while natural conversion from CBD could theoretically produce cis-Δ9-THC, this process would also lead to substantial amounts of Δ8- and Δ10-THC in Cannabis plants [Golombek et al. 2020], which is not observed. Therefore, in this analysis, trans:cis ratios > 8:1 are taken as evidence that the source of the THC is not hemp. If there is no cis-Δ9-THC in the sample, it is likely the THC is sourced from cannabis.
|
The quantity of Δ8-THC and Δ8-iso-THC
Δ8-THC occurs naturally in Cannabis sativa L., but only in negligible amounts. [Tagen and Klumpers 2022] Δ8-THC is formed during conversion from CBD to Δ9-THC [Marzullo et al. 2020], as is Δ8-iso-THC (along with its isomerized partner Δ4(8)-iso-THC). This “miscyclization” only presents itself in conversion reactions. This means that products created using naturally sourced Δ9-THC should have little to no Δ8-THC, with >1% Δ8-iso-THC+Δ8-THC (relative to the Δ9-THC amount) being taken as evidence of conversion from CBD via cyclization. [Marzullo et al. 2020]
Delineation of the Δ8-iso-THC and Δ8-THC amounts was not performed for this study, as the samples were run under standard UHPLC-DAD conditions for quantitation. The reported amounts of Δ8-THC, therefore, represent the combined contributions of both compounds.
The quantity of cannabigerol
The presence and quantity of CBG and other minor cannabinoids were also used as indicators of the source of the Δ9-THC. Just as the Δ8-THC in commercial products is produced via cyclization from CBD [Tagen and Klumpers 2022] because levels in “hemp” (i.e., Cannabis sativa L. plants with less than 0.3% Δ9-THC) are not naturally high enough to have a psychoactive effect, the low Δ9-THC levels in many hemp plants [Glivar et al. 2020; Johnson and Wallace 2021; Schafroth et al. 2021] may encourage manufacturers to use the same approach. Since CBD is the starting point for the cyclization reactions, the most efficient starting material is high-purity CBD “isolate,” which is not likely to have significant amounts of CBG present.
However, while natural hemp [Glivar et al. 2020; Schafroth et al. 2021] and cannabis [Radwan et al. 2021] contain minor cannabinoids such as CBG, this is not a product of the cyclization reaction. [Tagen and Klumpers 2022] If CBG is not present in the starting material nor a product of the conversion, it would not be expected to be present in converted Δ9-THC. Therefore, products with low quantities of CBG (<1% of total Δ9-THC content) are more likely to use converted Δ9-THC and those with higher quantities are more likely to use naturally sourced Δ9-THC.
Exact translation of fixed metrics for the Δ9-THC products in the study was not possible due to the wide range of Δ9-THC quantity in each product, but using the relative amounts of the three components along with some judgment calls allowed for grouping of each product into the three categories with reasonable confidence.
Age verification checks
Since all products were purchased from the companies’ websites, their use of age verification measures was considered. For each product, we noted if they required an ID to be presented on purchase or if an easily-circumvented method (e.g., simply entering a birth date) [Williams et al. 2020] was used. Additionally, we also noted how many products required an adult signature on delivery.
Packaging and labeling
The 53 products considered were inspected for warning labels, batch IDs, child-resistant containers, and the cannabis universal symbol (intended to alert consumers that the product contains large amounts of THC).
We define a warning label as a clear statement on the packaging that the product is intoxicating, is dangerous to minors and pets, or has specific situations in which you should not use the product, such as:
- For adults 18+, or 21+ where state law applies
- Will intoxicate, use extreme caution
- Keep away from children or pets
- Don’t drive after using
- Don’t operate heavy machinery
- Don’t consume if pregnant or breastfeeding
- Don’t consume if you are subject to drug testing
- Consult with your physician before use
Batch IDs are unique codes which identify a specific production batch of a product, thus enabling identification of other affected products in the event of contamination. These can have numerous formats, with an example from a purchased product being “E21362-10HC.”
Child-resistant containers are defined in law [Standards and (US) 1973] as those which 85% of children aged three to five cannot open within five minutes without a demonstration and which 80% still cannot open even after a demonstration. For example, a child-resistant cap may require the user to push down and twist the cap to open, rather than simply twisting. We did not test the packaging first-hand or verify that it met the legal definition, but took the presence of child-proofing mechanisms (such as a child-resistant cap) as evidence of a child-resistant container.
The cannabis universal symbol (Fig. 2) or some variation thereof is used to signify to consumers that the product contains cannabis and may be intoxicating. This is not required under the 2018 Farm Bill [Agriculture Improvement Act of (US) 2018], but it is required for high-THC products sold in adult-use markets such as California. [California Department of Cannabis Control: Requirements for Cannabis Goods 2022]
|
Lab reports provided by companies
In most cases, hemp consumers must depend on a certificate of analysis (COA) provided by the company itself to determine the true potency and safety of the product in question. These were also analyzed, in particular, whether they were tested for impurities or just potency, whether the lab used was ISO-accredited, and whether they were DEA-certified.
Results
Abbreviations, acronyms, and initialisms
Acknowledgements
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added.