Journal:A sustainable approach for the reliable and simultaneous determination of terpenoids and cannabinoids in hemp inflorescences by vacuum-assisted headspace solid-phase microextraction
| Full article title | A sustainable approach for the reliable and simultaneous determination of terpenoids and cannabinoids in hemp inflorescences by vacuum-assisted headspace solid-phase microextraction |
|---|---|
| Journal | Advances in Sample Preparation |
| Author(s) | Capetti, Francesca; Rubiolo, Patrizia; Mastellone, Giulia; Marengo, Arianna; Sgorbini, Barbara; Cagliero, Cecilia |
| Author affiliation(s) | Università di Torino |
| Primary contact | Email: cecilia dot cagliero at unito dot it |
| Year published | 2022 |
| Volume and issue | 2 |
| Article # | 100014 |
| DOI | 10.1016/j.sampre.2022.100014 |
| ISSN | 2772-5820 |
| Distribution license | Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International |
| Website | https://www.sciencedirect.com/science/article/pii/S2772582022000110 |
| Download | https://www.sciencedirect.com/science/article/pii/S2772582022000110/pdfft (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Cannabis sativa L. is an intriguing plant that has been exploited since ancient times for recreational, medical, textile and food purposes. The plant's most promising bioactive constituents discovered so far belong to the terpenoid and cannabinoid classes. These specialized metabolites are highly concentrated in the plant aerial parts, and their chemical characterization is crucial to guarantee the safe and efficient use of the plant material irrespective of which use it is.
This study investigates for the first time the use of vacuum-assisted headspace solid-phase microextraction (Vac-HSSPME) as a sample preparation process in an analytical protocol based on Vac-HSSPME combined to fast gas chromatography–mass spectrometry (GC-MS) analysis that aims at comprehensively characterising both the terpenoid and cannabinoid profiles of Cannabis inflorescences in a single step. The results proved that vacuum in the headspace should be preferred over atmospheric pressure conditions as it ensures the fast recovery of cannabinoid markers at relatively lower sampling temperatures (i.e., 90°C) that do not discriminate the most volatile fraction nor cause the formation of artifacts when the sampling time is minimized.
Keywords: Cannabis sativa inflorescences, vacuum-assisted headspace solid-phase microextraction, volatilome, terpenoids, cannabinoids
Introduction
Cannabis sativa L. currently can be considered one of the most studied plants due to its relevance in the illicit drug market and in the textile and food industry [1], as well as its potential medical usage. Whether the plant is intended for recreational purposes, fiber production (hemp), or medical use depends on the content of two major cannabinoids in the aerial parts of the plant: the psychoactive (-)-trans-Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD), the latter displaying several biological activities but not the psychotropic one. [2,3] The illicit drug chemotype, also known as type I, contains an excess of Δ9-THC and a limited amount of CBD. Contrary to that is the cannabis chemotype used in manufacturing (i.e., industrial hemp or type III), where the ratio is reversed and Δ9-THC content cannot exceed 0.2%. Finally, the type II chemotype, which is used for medical purposes, is defined as having high mean contents of both CBD and Δ9-THC (i.e., Bedrocan: 22% THC, <1% CBD; Bediol: 6.5% THC, 8% CBD). [3][4][5]
Other than Δ9-THC and CBD, the plant may synthesize several specialised metabolites, including additional phytocannabinoids and terpenes, amongst others [6], which are both produced by stalked glandular trichomes that are highly concentrated on female inflorescences. [7] Phytocannabinoids are C21 compounds known as terpenophenolic compounds. They are produced by the plant in their acidic form, which under heating or during storage is decarboxylated into the active neutral form. [6] At least 104 cannabinoids have been isolated so far. [8] Aside from Δ9-THC, CBD, cannabichromene (CBC), and cannabigerol (CBG), other predominant secondary metabolites are Δ9-tetrahydrocannabinolic acid (Δ9-THCA), cannabidiolic acid (CBDA), cannabichromenic acid (CBCA), and cannabigerolic acid (CBGA), which are the precursors of the formerly mentioned compounds. Other minor cannabinoids include cannabinolic acid (CBNA) and Δ8THCA, which are artifacts of Δ9THCA, as well as cannabielsoic acid (CBEA) and cannabinodiolic acid (CBNDA), which derive from CBDA. [9]
Terpenes are cannabis's most abundant specialized metabolites, including at least 120 identified terpenoids. [8] Literature data suggest that varying pharmaceutical properties between different Cannabis varieties can be attributed to synergistic interactions, known as the "entourage effect," between cannabinoids and terpenes. [9] A comprehensive qualitative characterisation of both the cannabinoid and terpene profiles of the plant raw material is therefore of utmost importance not only to define its rational use (i.e., whether the plant under investigation was cultivated for fiber production, medical, or drug purposes) but also to guarantee the efficacy and safety of its potential pharmaceutical application.
The most employed method of extraction of cannabinoids from plant raw material is solid-liquid extraction (SLE) using ethanol or acetone as extracting solvents due to their affinity and consequent high extracting efficiency for cannabinoids. [10,11] High-performance liquid chromatography (HPLC) and gas chromatography coupled to mass spectrometry (GC-MS) are the analytical techniques of choice for many qualitative and quantitative analyses. [11] HPLC is usually employed when the acid and the neutral form of the investigated cannabinoids must be measured separately, while GC analyses enable the characterization of the total cannabinoid content (e.g. the combined amount of THC and THCA), as GC systems, by definition, work with high temperatures that lead unavoidably to the decarboxylation of cannabinoid acids. [11] The total cannabinoid content is usually measured as it best represents the pharmacological activity of the material, unless differently stated by legislation. [12]
Thanks to their volatile nature, the isolation of terpenes—and in particular of mono- and sesquiterpenes—from plant raw material is straightforward, and their profiling can be performed by headspace solid-phase microextraction (HSSPME) online combined to GC-MS analysis [5].
The recovery of cannabinoids from solid matrices by HSSPME is also feasible but requires long sampling times in combination to high sampling temperatures due to their low volatility and low tendency to escape to the headspace. In 2004, Lachenmeier et al. optimized a successful HSSPME method followed by on-coating derivatization of the cannabinoids with N-methyl-N-trimethylsilyltrifluoroacetamide (MSTFA) for the extraction of cannabinoids from hemp food products using 90°C as sampling temperature and 30 minutes of extraction time [13], while in 2005, Ilias et al. showed that cannabinoid extraction should be performed at 150°C to maximize their recovery in short sampling times (e.g., five minutes). [14] However, in more recent work, Czégény et al. investigated the effect of temperature on the composition of pyrolysis products of CBD in e-cigarettes. They tested different operating temperatures (250–400°C), and they proved that, depending on the temperature and atmosphere (i.e., inert of oxidative condition), 25–52% of CBD can be converted into other cannabinoids, amongst which Δ9-THC, Δ8-THC, cannabinol (CBN), and CBC are the predominant pyrolysates. [15] Even though when performing HSSPME it is usually unlikely to reach such extreme temperatures, the results of Czégény et al. suggest that (1) reduced sampling temperature should be preferred to obtain a truthful cannabinoid fingerprint profile in the plant raw material, and (2) CBD potential degradation should be investigated during the optimization of the sampling temperature.
As thoroughly described by Psillakis et al. [16][17][18][19], vacuum is a powerful experimental parameter to consider for increasing the extraction kinetic of semi-volatile compounds during the HSSPME process. This is because in the case of semi-volatiles and under non-equilibrium conditions, a reduced pressure inside the sample container decreases the resistance to mass transfer in the gas zone at the solid-headspace interface. As a consequence, higher extraction efficiencies for semi-volatile compounds can be achieved in shorter sampling times, and potentially at milder extraction temperatures. [20,21]
This study investigates the advantages and disadvantages of using vacuum-assisted headspace solid-phase microextraction (Vac-HSSPME) over HSSPME as a sample preparation process to be exploited in analytical protocols, aiming to comprehensively characterize both the terpene and cannabinoid profiles of Cannabis inflorescences in a single step while employing a total analysis system.
Materials and methods
Chemicals and samples
CBD and CBC standard solutions 1.0 mg mL−1 in methanol were purchased from Merck KGaA, Darmstadt, Germany. Dried Cannabis inflorescences of type III chemotype were purchased from an authorized local hemp shop. The dried plant material was pulverized by an electric blender and stored at -18°C.
Procedures under reduced (Vac-HSSPME) and atmospheric pressure conditions (HSSPME)
Divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) 50/30 μm (2 cm length) and over coated PDMS/DVB 75 μm (coating thickness includes 65 μm coating + 10 μm OC [overcoating]) fibers were employed for the experiments. The fibers were purchased from Merck KGaA, Darmstadt, Germany and conditioned following the manufacturer's instructions.
HSSPME experiments were performed using conventional headspace 20 mL crimp vials provided by Restek, Bellefonte, USA, as well as a 20 mm magnetic ring crimp cap, fitted with 20.6 mm septa (Buty red/PTFE grey, 55 shore A, 1.3 mm).
Vac-HSSPME experiments were performed in the same commercial headspace 20 mL crimp vials but hermetically sealed with a stainless steel closure (provided by Prof. Elefteria Psillakis) having a hole that could tightly accommodate a Thermogreen LB-1 septum with half-hole (Supelco, Bellefonte, USA), through which the air evacuation step and the SPME sampling were performed.
For the inflorescences, 10 mg of the pulverized sample were placed inside the vial, which was again stored at −18°C for one hour and then air-evacuated. [20] The air-evacuation step was performed with a 22-gauge hypodermic needle sealed to a 5 mL syringe tightly secured to the tubing of a N 820.3 FT.18 (7 mbar ultimate vacuum) pumping unit manufactured by KNF Lab (Milan, Italy). The needle was inserted through the septum, and the vial was air-evacuated for one minute. The procedure was the same for HSSPME, while omitting the air evacuation step.
For CBD standard sampling, 10 µL of the 1.0 mg mL−1 solution were introduced through the closure septum after the air-evacuation step. For HSSPME experiments, the liquid sample was introduced into the vial by the open-vial technique. [22]
After sampling, the fiber was withdrawn and the SPME device moved to the GC-MS system for analysis. GC desorption lasted 10 minutes to minimize carry-over.
Ten milligrams of pulverized plant material were sampled at three different extraction temperatures (i.e., 80, 90 and 150°C) under both Vac-HSSPME and HSSPME experiments. Sampling-time profiles were obtained for all the above mentioned conditions by sampling for 5, 15, and 30 minutes. 10 µL of CBD standard solution 1.0 mg mL−1 were sampled at 90 and 150°C for 5 minutes, under both pressure conditions. All extractions were run in triplicate.
Instrument set-up
GC-MS systems and columns
Analyses were carried out on two different instruments: (1) a MPS-2 multipurpose sampler (Gerstel, Mülheim a/d Ruhr, Germany) installed on a Shimadzu gas chromatography–flame ionization detector–mass spectrometry (GC-FID-MS) system, consisting of a Shimadzu GC 2010 system, equipped with FID, in parallel with a Shimadzu QP2010-PLUS GC–MS mass spectrometer (Shimadzu, Milan, Italy); (2) a MPS-2 multipurpose sampler (Gerstel, Mülheim a/d Ruhr, Germany) installed on an Agilent 6890 N GC system coupled to a 5975 MSD mass spectrometer (Agilent Technologies, Santa Clara, CA).
GC analyses were carried out using two MEGA-5 95% methyl-polysiloxane 5%-phenyl (MEGA, Legnano, MI, Italy) columns: a conventional 30 m × 0.25 mm dc, 0.25 µm df column installed on the Agilent 6890 N GC - 5975 MSD, and a narrow-bore 15 m × 0.18 mm dc, 0.18 µm df column installed on the Shimadzu GCMS-QP2010.
Data was processed with the ChemStation Version E.02.02.1431 data processing system (Agilent Technologies, Santa Clara, CA).
GC-MS conditions
Analyses were carried out under the following conditions. Temperatures: injector: 250 °C, transfer line: 270 °C, ion source: 200 °C; carrier gas: He; flow control mode: constant linear velocity; flow rate: 1.00 mL min−1 (conventional column), 0.72 mL min−1 (narrow bore column); injection mode: split; split ratio: 1:20.
The MS was operated in electron ionization mode (EI) at 70 eV, scan rate: 666 u/s, mass range: 35–350 m/z. Temperature programs: (i) 50°C (one minute)// 3°C/min//250°C (five minutes) for conventional MEGA-%; (ii) 50°C (30 seconds) //7.2°C/min// 250°C (two minutes) for the narrow-bore column. The chromatographic conditions for the narrow-bore columns were obtained by translating the method parameters through the Agilent method translator software. [23] Identification was performed via comparisons of linear retention indices and mass spectra either with those of authentic standards, or with data stored in commercial [24] and in-house libraries.
Results and discussion
Table S1 in the Supplementary materials provides the list of the target compounds together with their physicochemical properties (i.e., LogKow, boiling point, and vapor pressure). The mono- and sesquiterpene markers to be investigated were chosen according to the results of Jin et al., who comprehensively profiled reference specialized metabolites in Cannabis inflorescences, leaves, stem barks, and roots for the three Cannabis chemotypes (i.e., Type I, II and III). [8]
Preliminary optimization of the fiber coating and chromatographic conditions
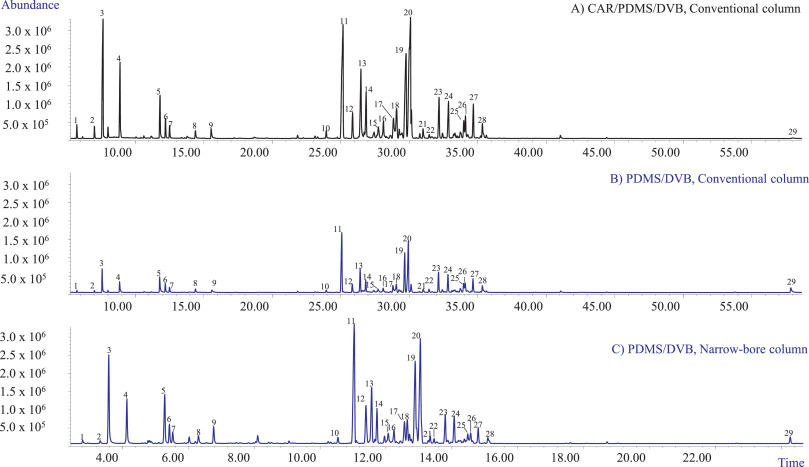
The first set of experiments aimed at selecting (1) the optimum fiber coating that could extract all the investigated analytes with acceptable sensitivity and (2) the best chromatographic conditions providing an acceptable resolution of all the investigated markers in a reasonable time for high-throughput analyses. In our study, two fiber coatings were tested: the PDMS/DVB and the DVB/CAR/PDMS fibers. Fig. 1A and B show the profiles obtained with the two investigated coatings when sampling 10 mg of matrix with HSSPME at 90°C for 30 minutes of extraction. The chromatographic analyses were performed employing a conventional 30 m × 0.25 mm dc, 0.25 µm df MEGA-5 column. Irrespective of the fiber coating, the most abundant compounds amongst the recovered mono- and sesquiterpenes were β-myrcene (3), trans-β-caryophyllene (11), and selina-3,7(11)-diene (20), while only one cannabinoid—CBD (29)—was recovered. In agreement with the results of Ilias et al. [14], the PDMS/DVB fiber proved to be more efficient for the recovery of CBD compared to the triphasic fiber which, however, extracted a quantitative richer mono- and sesquiterpene profile. The PDMS/DVB fiber was chosen for the subsequent experiments because of its higher recovery of CBD while still providing an acceptable sensitivity for the investigated mono- and sesquiterpene metabolites.
The employed conventional MEGA-5 column proved to separate the main selected markers with an acceptable resolution. The possibility to improve the analysis speed and to decrease the use of gas with a greener analytical protocol was then investigated. The chromatographic analysis was sped up by translating the method to a 15 m × 0.18 mm dc, 0.18 µm df column using the method translation approach. [23,25,26] Fig. 1C reports the translated GC-MS profile of the investigated hemp inflorescences with the narrow-bore MEGA-5 column. The analysis time was reduced from 72.67 to 30.28 minutes, while maintaining the separation and the elution pattern of the investigated markers.
|
Abbreviations, acronyms, and initialisms
- Δ9-THC: Δ9-tetrahydrocannabinol
- Δ9-THCA: Δ9-tetrahydrocannabinolic acid
- CBC: cannabichromene
- CBCA: cannabichromenic acid
- CBD: cannabidiol
- CBDA: cannabidiolic acid
- CBEA: cannabielsoic acid
- CBG: cannabigerol
- CBGA: cannabigerolic acid
- CBN: cannabinol
- CBNA: cannabinolic acid
- CBNDA: cannabinodiolic acid
- GC: gas chromatography
- GC-FID-MS: gas chromatography–flame ionization detector–mass spectrometry
- GC-MS: Gas chromatography–mass spectrometry
- HPLC: high-performance liquid chromatography
- HSSPME: headspace solid-phase microextraction
- SLE: solid-liquid extraction
- SPME: solid-phase microextraction
- Vac-HSSPME: vacuum-assisted headspace solid-phase microextraction
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. Nothing else was changed in accordance with the NoDerivatives portion of the license.