Journal:A spectroscopic study to assess heavy metals absorption by a combined hemp-spirulina system from contaminated soil
| Full article title | A spectroscopic study to assess heavy metals absorption by a combined hemp-spirulina system from contaminated soil |
|---|---|
| Journal | Environmental Advances |
| Author(s) | Musio, Biagia; Ahmed, Elhussein M.F.M.H.; Antonicelli, Marica; Chiapperini, Danila; Dursi, Onorfrio; Grieco, Flavia; Latronico, Mario; Mastrorilli, Piero; Ragone, Rosa; Settanni, Raffaele; Triggiani, Maurizio; Gallo, Vito |
| Author affiliation(s) | Polytechnic University of Bari, Innovative Solutions S.r.l., International Centre for Advanced Mediterranean Agronomic Studies of Bari, ApuliaKundi S.r.l. |
| Primary contact | Email: vito dot gallo at poliba dot it |
| Year published | 2022 |
| Volume and issue | 7 |
| Article # | 100144 |
| DOI | 10.1016/j.envadv.2021.100144 |
| ISSN | 2666-7657 |
| Distribution license | Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International |
| Website | https://www.sciencedirect.com/science/article/pii/S2666765721001150 |
| Download | https://www.sciencedirect.com/science/article/pii/S2666765721001150/pdfft (PDF) |
Abstract
The efficiency of hemp (Cannabis sativa L.) in remediating sites contaminated with heavy metals has received great attention in recent years. The main advantage of this technology relies on its inherent sustainability, with a potential re-utilization of the significant amount of produced biomass, which acts as a valuable flow resource. In this study, a combined system consisting of Cannabis sativa L. (hemp) and the blue-green alga Arthrospira platensis (spirulina) was tested to clean up soils contaminated with cadmium, chromium, copper, nickel, lead, and zinc. The application of non-targeted nuclear magnetic resonance spectroscopy (NMR) methods combined with inductively coupled plasma atomic emission spectroscopy (ICP-AES) quantification provided an efficient strategy for detecting residual heavy metals within plant tissues and soil. Importantly, non-targeted metabolomic analysis helped to reveal the relationships between metabolites distribution in hemp tissues and the sequestered metals. It was demonstrated that hemp accumulates copper, chromium, nickel, and zinc preferentially in the leaves, while lead is distributed mainly in the stems of the plant. Moreover, it was found that, at higher concentrations, spirulina acts as a growth promoter, contributing to an increase in the final generated biomass. Results reported in this work indicate that the hemp-spirulina system represents a suitable tool for remediation of metal contaminated soils by modulating biomass production and metals uptake.
Keywords: non-targeted nuclear magnetic resonance, phytoremediation, phycoremediation, Arthrospira platensis, Cannabis sativa L., metal quantification
Graphical abstract:
Introduction
Dispersion of heavy metals in soils is an age-old problem deriving from both natural and anthropic sources.[1] Among the anthropic contribution to soil contamination by metals, land application of treated wastewater, sewage sludge, fertilizers, and industrial activities are major concerns.[2] Unbalanced amounts of heavy metals may cause perturbation of soil parameters with consequent toxic effects on plants, in the nearby water supplies, and, ultimately, in the whole food chain.[3][4][5] Typically, elements such as copper (Cu), nickel (Ni), zinc (Zn), and chromium (Cr) are biologically essential for plant growth but become toxic for animals and plants when their concentrations exceed certain threshold levels.[6][7][8] Other heavy metals often found in contaminated soils, such as cadmium (Cd) and lead (Pb) are not essential for plants growth, and many studies have associated their presence with neurological and endocrinological toxicity for humans, along with carcinogenic effects.[9][10][11]
Since heavy metals are not biodegradable, they tend to accumulate in the environment, becoming a high risk for biota over several years after their introduction in an ecosystem.[12][13][14] The search for new solutions that can remediate soil contaminated by heavy metals is a critical prerequisite for the sustainable development of agriculture[6][15][16], thus representing a topic of paramount importance. The most consolidated strategies to remediate such contaminated soils include physical and chemical approaches like isolation, through capping and subsurface barriers; immobilization, by solidification/stabilization, vitrification, and chemical treatment; physical separation; and extraction, by soil washing, pyrometallurgical extraction, in situ soil flushing, and electrokinetic treatment.[17][18][19][20] However, alternative approaches are gaining greater attention as they combine cost-effectiveness, sustainability, low toxicity, and mobility decrease. They include bioaccumulation, phytoremediation (e.g., phytoextraction, phytostabilization, and rhizofiltration), bioleaching, and other biochemical processes in which living organisms such as plants or microbes are used to clean contaminants from an area.
In particular, phytoremediation is attracting the attention of the scientific community, since it has been demonstrated to be a cost-effective solution for the remediation of contaminated sites, and, in the meantime, to be a feasible method for bio-fixation of CO2, resulting in highly sustainable technology.[1] The ability to absorb heavy metals generally depends on the biomass produced, as well as on the ability of the plant to accumulate and translocate heavy metals in its biomass.[21][22][23] According to recent scientific literature, a good candidate for phytoremediation of soil contaminated by heavy metals is the hemp plant.[24][25][26] Kompolti, also known as hemp, the non-psychoactive variety of Cannabis sativa L., is an annual dioecious high-yielding industrial crop, and it is mainly grown for its fibers and seeds, generally being used for textiles, clothing, insulation, biodegradable plastics, food, animal feed, and biofuel production.[27][28][29][30] Hemp possesses some characteristics that make it quite suitable for phytoremediation, such as high biomass, long roots, and a favorably short industrial life cycle of 180 days. Importantly, hemp demonstrates a strong capability to sequester heavy metals like cadmium, zinc, lead, nickel, copper, and chromium when they are present in contaminated soil and water.[26][31][32][33]
Another attractive approach for the remediation of contaminated sites is the application of bioleaching technology, which uses direct metabolism or by-products of microbial processes to uptake heavy metals adsorbed onto the soil surface and to transform them so that the elements can be extracted when water is filtered through. Bioleaching has several advantages over conventional physical and chemical strategies, such as low cost, environmental sustainability, few hazardous characteristics of waste/sludge, low energy demand, and absence of toxic chemicals.[34][35][36][37][38][39]
Additionally, phycoremediation, which involves eukariotic algae and cyanobacteria in remediation processes, has been extensively applied to the treatment of wastewater.[1] However, its application to the remediation of sediments and soils contaminated by heavy metals is less documented. Among the cyanobacteria, Arthrospira platensis possesses excellent chelating properties both towards heavy metals present in humans and towards those present in soil, water, and sludge.[40][41][42][43][44][45] The dried biomass of Arthrospira platensis is commonly known as "spirulina," and it finds many applications in agriculture as a plant growth promoter, enhancing growth, increasing yield, and speeding up seed germination.[46][47] Recently, the employment of this blue-green alga to uptake heavy metals in contaminated sites has been explored.[47][48] The presence of a chloroplast-type ferredoxin in the active center has been reported as responsible for the chelating capability of spirulina[49], whereby its efficiency is affected by many physical and chemical factors such as initial metal concentration, dosage, adsorption time, temperature, and pH.[50]
The present study aims at both exploring the ability of the unreported combined use of hemp and spirulina to uptake six selected heavy metals (Cd, Ni, Cr, Pb, Cu, Zn) from artificially contaminated soil and investigating, under controlled plant growing conditions, their distribution into the plant tissues. Specifically, hemp was chosen as the main agent for biological remediation, and spirulina was added as an enhancer of both the plant growth and the translocation of heavy metals in the hemp. The application of a non-targeted nuclear magnetic resonance spectroscopy (NMR) approach combined with an estimation of the residual metals by inductively coupled plasma atomic emission spectroscopy (ICP-AES) into the cultivation soil and within the different tissues of the plant was applied in view of gathering useful information on the efficiency of the integrated hemp-spirulina system. Obtaining this information is crucial for the potential re-utilization of the hemp plant or shoots of it, after the phytoremediation stage, for alternative usages, like production of bio-materials for the textile, construction, and bio-fuel industries.
Materials and methods
Materials
3-(Trimethylsilyl)-2,2,3,3-tetradeutero-propionic acid sodium salt (TSP, CAS N. 24493-21-8, 99 %D, Armar Chemicals, Döttingen, Switzerland), hydrochloric acid (HCl, 37%, CAS N. 7647-01-0; ≥ 99.5%, Sigma-Aldrich, Milan, Italy), sodium oxalate (Na2C2O4, CAS N. 62-76-0; ≥99.5%, Sigma-Aldrich, Milan, Italy), sodium azide (NaN3, CAS N. 26628-22-8; ≥99.5%, Sigma-Aldrich, Milan, Italy), and deuterium oxide (D2O, CAS. N. 7789-20-0, 99.86 %D, Eurisotop, Saclay, France) were used for sample preparation. NMR tubes (Norell 509-UP 7) were provided by Norell, Landisville NJ, United States. The NMR samples were prepared using an automated system for liquid handling (SamplePro Tube, Bruker BioSpin).
The soil used during cultivation was Plagron Lightmix (Plagron, Ospel, The Netherlands), which had pH 6-7, electric conductivity (E.C. 1:5) 310-470 µS•cm−1 and (E.C. 1:1.5) 0.7-1.1 µS•cm−1, NPK (12-14-24) 1.5 kg•m−3, total N 180 g•m−3 (105:75, NO3:NH4), P2O5 210 g•m−3, K2O 360 g•m−3, dry matter 37% (of which 82% organic matter), and water retention of 6 mL•g−1 dry matter.
The soil was contaminated by Cd(NO3)2‧4H2O (CAS N. 10022-68-1, Carlo Erba Reagents, Milan, Italy), K2Cr2O7 (CAS N. 7778-50-9, Carlo Erba Reagents, Milan, Italy), Cu(SO4)‧5H2O (CAS N. 7758-99-8, Prolabo, Paris, France), Pb(CH3COO)2 (CAS N. 301-04-2, Carlo Erba Reagents, Milan, Italy), Ni(NO3)2‧6H2O (CAS N. 13478-00-7, Carlo Erba Reagents, Milan, Italy), Zn(CH3COO)2‧2H2O (CAS N. 5970-45-6, Carlo Erba Reagents, Milan, Italy).
Hydrochloric acid (HCl 37%, Merck, Darmstadt, Germany), nitric acid (HNO3 65%, Merck, Darmstadt, Germany), double-distilled water (DDW ≥ 99.5%), standard multi-elemental reference solution (Ag, Al, Ba, Be, Bi, Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, Pb, Sr, Tl, V, Zn [10 mg/l]) were used for heavy metals analysis by ICP-AES.
Algal strain and growth conditions
An Arthrospira platensis strain was cultivated by ApuliaKundi S.r.l. in open ponds (3,000 L per pond) under controlled greenhouse conditions with natural light at a temperature ranging from 22 to 28 °C.
The production cycle was monitored by turbidity measurements through a Secchi disk (200 mm diameter, Scubla, Remanzacco (UD), Italy). Once the disk was lowered into the algae suspension at 5÷6 cm and could not be seen anymore, the algae were collected. Overall, the production cycle lasted approximately three days. The collected algal biomass was filtered (40 μm filter), extruded, and cold dried at a temperature value lower than 38 °C. The study was conducted using the dried biomass in powder form, commonly named "spirulina."
Cultivation of Cannabis sativa L. (cv. Kompolti)
The cultivation of hemp by Enjoy Farm Soc. Coop. (Bitetto, Bari, Italy) took place indoors with controlled light and temperature conditions using a vegetative photoperiod of 18/6 h of light/dark for the first nine weeks, and a flowering photoperiod of 12/12 h of light/dark until harvest.[51] Germination occurred 10 days after the seed sowing. After 50 days from germination, six plants were transplanted into 15 L pots filled with four kilograms of 75-days-old contaminated soil, in addition to two control plants transplanted into pots filled with four kilograms of uncontaminated soil. The pre-contaminated soil was prepared upon controlled addition into the soil of six different heavy metals, namely cadmium (Cd), chromium (Cr), copper (Cu), lead (Pb), nickel (Ni), and zinc (Zn). The choice of heavy metals and quantities used for contamination was based on the typical content of metals detected in different sites of the Apulia region of southern Italy, as provided by Agenzia Regionale per la Prevenzione e la Protezione dell'Ambiente (ARPA Puglia) and Acquedotto Pugliese S.p.A. Specifically, the pre-contaminated soil contained 16, 527, 200, 434, 357, and 448 mg•kg−1 of Cd(NO3)24H2O, K2Cr2O7, Cu(SO4)2‧5H2O, Pb(CH3COO)2, Ni(NO3)2‧6H2O, and Zn(CH3COO)2‧2H2O, respectively. Commercial uncontaminated soil (24 kilograms) was treated with 2.0 L of such salts aqueous solutions through four subsequent additions. The resulted mixture was stirred in a concrete mixer for three hours. Finally, the obtained contaminated soil was distributed into six pots (15 L each) and stored in the greenhouse under controlled conditions for 75 days.
Moreover, four out of the six Cannabis sativa L. plants grown in pre-contaminated soil received irrigation water (0.50 L of water × 56 times) containing spirulina (added in powder form). As a proof of concept, two different concentrations were tested: 1.0 and 0.50 g•L−1. Overall, each experimental condition was tested in duplicate, involving a total of eight hemp plants (Table 1).
| |||||||||||||||||
Plants were constantly monitored until the harvest (over two months after the sowing) by recording three main morphometric parameters: (i) plant height (cm), i.e., height of stem from ground to apex; (ii) the number of leaf stages; and (iii) drum diameter (mm) (see Supplementary materials for the full list of the morphometric parameters, Table S1). The biomass (g) of stem and leaf samples was determined upon lyophilization; the sum of the biomass of the stem and leaf samples collected from the same plant was computed too and is herein referred to as “plant biomass.” The residual amount of heavy metals (mg•kg−1) contained in leaves, stems, and soil after the harvest was measured; for each plant, also the sum of the heavy metal content of leaves plus stems was calculated (see Supplementary materials for further details, Table S2). The shoots of plants were collected and transferred in refrigerated packaging with dry ice from the greenhouse to laboratories for further analyses. Samples of soil from each pot were collected after harvesting and kept in plastic bags at room temperature until analysis. Leaves and flowers were separated from stems, and both portions of the plant were firstly freeze-dried at –50 °C and 0.180 mbar for 72 hours in a lyophilizer (Christ Alpha 1–4 LSC, Martin-Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany). Then, the dried samples were mechanically ground in a blender, sieved through a mesh with a pore size of 0.5 mm, and stored at –20 °C until analysis.
Sample preparation and NMR analysis
An aliquot of 50 mg of each powdered sample was dissolved in 1.5 mL of buffer solution [Na2C2O4 (0.25 M), NaN3 (2.5 mm)] at pH 4.2 [pH adjusted by addition of HCl (37%)], sonicated for 10 minutes at 40 kHz, and vortexed (Advanced Vortex Mixer ZX3, VELP Scientifica Srl, Italy) for five minutes at 2,500 rpm. The mixture was transferred in a centrifuge tube (2 mL Nonsterile Centrifugal Filters, ThermoFisher Scientific) equipped with a polytetrafluoroethylene (PTFE) filter (0.2 µm) and centrifuged for 15 minute at 4,700 rpm (ROTOFIX 32 A, Hettich, Italy). The filtrate was used to fill the NMR tube.
NMR tubes were filled in by an automated system for liquid handling (SamplePro Tube, Bruker BioSpin) according to the following method: 630 µL of the obtained extract and 70 µL of TSP/D2O solution [3-(trimethylsilyl) propionic-2,2,3,3-d4 acid sodium salt in D2O (0.2% w/w)]. Three replicates were prepared for each sample.
One-dimensional 1H NOESY NMR spectra were recorded through a Bruker Avance 400 MHz spectrometer equipped with a 5 mm inverse probe and with an autosampler. The following acquisition parameters were used: pulse program = noesygppr1d; size of fid (TD) = 65 K; spectral width (SW) = 20 ppm; transmitter offset = 4.70 ppm; 90 ° hard pulse (p1) = 12.50 μs; power level for pre-saturation (pl9) = 59.10 dB; dummy scans (ds) = 4; number of scans (ns) = 128; acquisition time = 4.09 seconds; mixing time (d8) = 0.01 seconds; recycle delay (d1) = 5 seconds. Each spectrum was acquired using TOPSPIN 2.1 software (Bruker BioSpin GmbH, Rheinstetten, Germany) under an automatic process that lasted ca. 22 minutes and encompassed sample loading, temperature stabilization for five minutes, tuning, matching, shimming, and 90 ° pulse calibration.
NMR raw data (Free Induction Decays, FIDs) were processed using the software MestReNova 11.0 (Mestrelab Research SL, Santiago de Compostela, Spain). The FIDs relative to 48 1D 1H NOESY NMR experiments (48 experiments composed as follows: 8 leaf samples × 3 replicates, 8 stem samples × 3 replicates) were zero-filled with 128 K number of points and then underwent to Fourier transformation by applying an exponential multiplication function with a line broadening of 0.1 Hz. Phase and baseline were automatically corrected, and the TSP singlet signal set at δ = 0.00 ppm was used as a chemical shift reference.
ICP-AES analysis for quantification of heavy metals
Quantitative analysis of metals in samples of soil, stem powder, and leaf powder was carried out through ICP-AES, in three technical replicates. ICP-AES analyses were carried out at SAMER (Special Agency of the Chamber of Commerce of Bari). Samples of 200 mg were weighed into 100 mL digestion vessels using bi-distilled water, followed by acid digestion using 6 mL of 37% HCl (Merck, Darmstadt, Germany) and 2 mL of 65% HNO3 (Merck, Darmstadt, Germany). Digestion vessels were closed and placed into CEM Mars 1 microwave oven (CEM Corporation, Matthews, NC, USA). Power and time parameters used in the present work are part of an optimized library program for the analysis of trace metals in soil and plant material. The following digestion program has been, thus, selected for our purpose: 250 W (for two minutes), 0 W (for two minutes), 250 W (for five minutes), 400 W (for five minutes), and 500 W (for five minutes). After cooling to room temperature, the samples were filtered through 0.45 µm porosity filters and brought to 10 mL volume using bi-distilled water.
The quantitative measures of the metals were performed by ICP-AES (iCAP 6300 Duo, Thermo Fisher Scientific, Bremen, Germany). Quantities were reported in mg•kg−1. The amounts of Cd, Cr, Cu, Ni, Pb, and Zn were determined according to the UNI EN ISO 13657:2004 and UNI EN ISO 11885:2009 standards. The working wavelengths of the analyzed elements were 226.5 nm (Cd), 267.7 nm (Cr), 324.7 nm (Cu), 231.6 nm (Ni), 220.3 nm (Pb), and 213.8 nm (Zn). A concentrated standard reference solution was used at a concentration of 10 mg•kg−1 of Cd, Cr, Cu, Ni, Pb, and Zn. The six elements indicated, taken from 1000 mg•kg−1 solutions, were brought in a single solution from 100 mg•kg−1. Dilutions of 1, 0.5, 0.1, 0.01, 0.005, and 0 mg•kg−1 of the concentrated standard reference solutions were made in final volumes of 100 mL using HNO3 and HCl.
A calibration blank was used to obtain the analytical curve and was prepared by acidifying water with a mixture of 65% HNO3 and 37% HCl in a way that the standard and the sample reached the same value of acidity. Furthermore, the method blank contained all reagents in the same volumes used in the sample preparation and was used to identify possible contamination resulting from the acidic reagents used or equipment used during the sample preparation, including the filtration step. The calibration standard analytical curve was made of 1.0, 0.50, 0.10, 0.010, 0.0050, and 0 mg•kg−−1.
Statistical elaboration of NMR and ICP-AES data
The 48 processed 1D 1H NOESY spectra (8 leaf samples × 3 replicates, 8 stem samples × 3 replicates) were reduced to a numerical matrix (bucket-table) manageable for multivariate statistical analysis. The bucket-table was obtained by dividing the entire spectrum in the range of [-0.514, 10.486] ppm into 249 rectangular intervals (buckets) of 0.04 ppm in width, excluding the region [5.126, 4.566] ppm corresponding to the residual water signal. The underlying area of each bucket was normalized to the total intensity. The bucket table was imported into SIMCA 13.0.3 software (Umetrics, Umea, Sweden) to perform multivariate statistical analyses (MVA). The coordinates of the observations in the new space are called "scores," and the weight of the original variables on each PC are called "loadings." In the present study, the NMR spectra constituted the observations and the buckets constituted the x-variables. Buckets were centered and subjected to Pareto scaling (eachxj -variable was scaled to 1/sqrt(sdj), where sdj is the standard deviation ofxj -variable computed around the mean) to avoid noise inflation. The buckets in the range [-0.514, 0.486] ppm, including the TSP singlet, were excluded from statistical analysis.
Data from ICP-AES analysis and morphometric data were also included in statistical analysis as y-variables and were scaled to unit variance (UV), as recommended for variables expressed in different units (for eachyj -variable the base weight is computed as 1/sdj, where sdj is the standard deviation of yj-variable computed around the mean). Principal component analysis (PCA) and orthogonal partial least squares (OPLS) analysis were performed. The quality of statistical models was assessed based on the parameters R2 and Q2, which represent the descriptiveness (goodness-of-fit) and the predictivity in cross-validation (goodness-of-prediction in cross-validation), respectively. For OPLS models, permutation tests were carried out to verify the absence of model overfitting.
Results and discussion
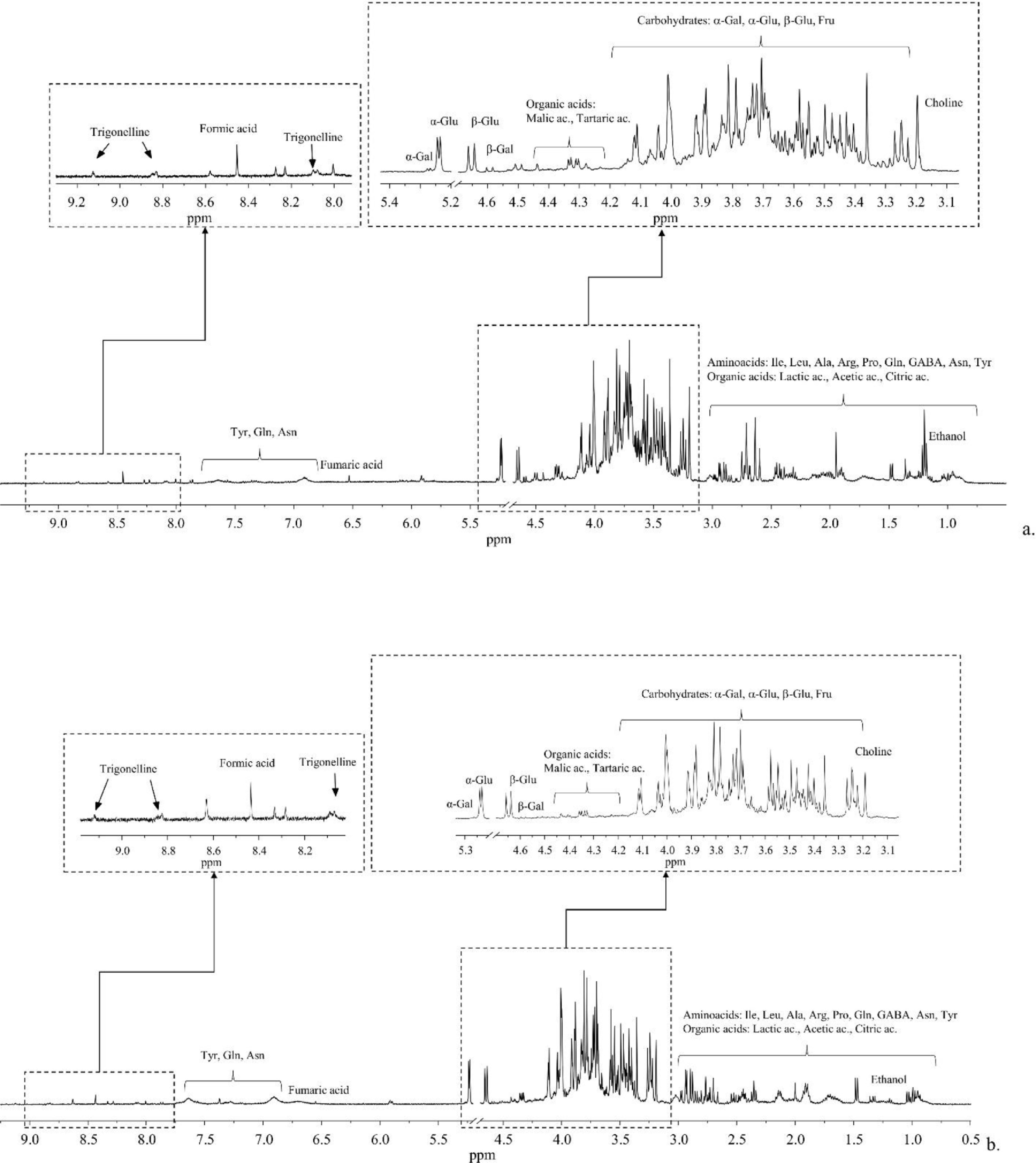
NMR analysis (1D 1H NOESY experiments) allowed for the identification of a pool of metabolites contained in typical aqueous extracts (pH = 4.2) of leaves (Fig. 1a) and stems (Fig. 1b) collected from a plant of hemp cultivated under controlled conditions in uncontaminated soil (see Supplementary materials for the full list of metabolites, Table S3). The main representative classes of metabolites included organic acids (i.e., lactic, citric, tartaric, fumaric, formic, and acetic acids), amino acids (i.e., alanine, asparagine, γ-aminobutyric acid, arginine, glutamine, isoleucine, proline, tyrosine, leucine), and carbohydrates (i.e., β-glucose, α-glucose, fructose, α-galactose, β-galactose). Moreover, 1D 1H NOESY spectra also contained signals related to trigonelline, choline, and ethanol.
|
A holistic approach was applied during the present study by combining the information provided by the 1D 1H NOESY experiments, the ICP-AES quantitative analysis of residual metal, and the data derived from the morphometric inspection.
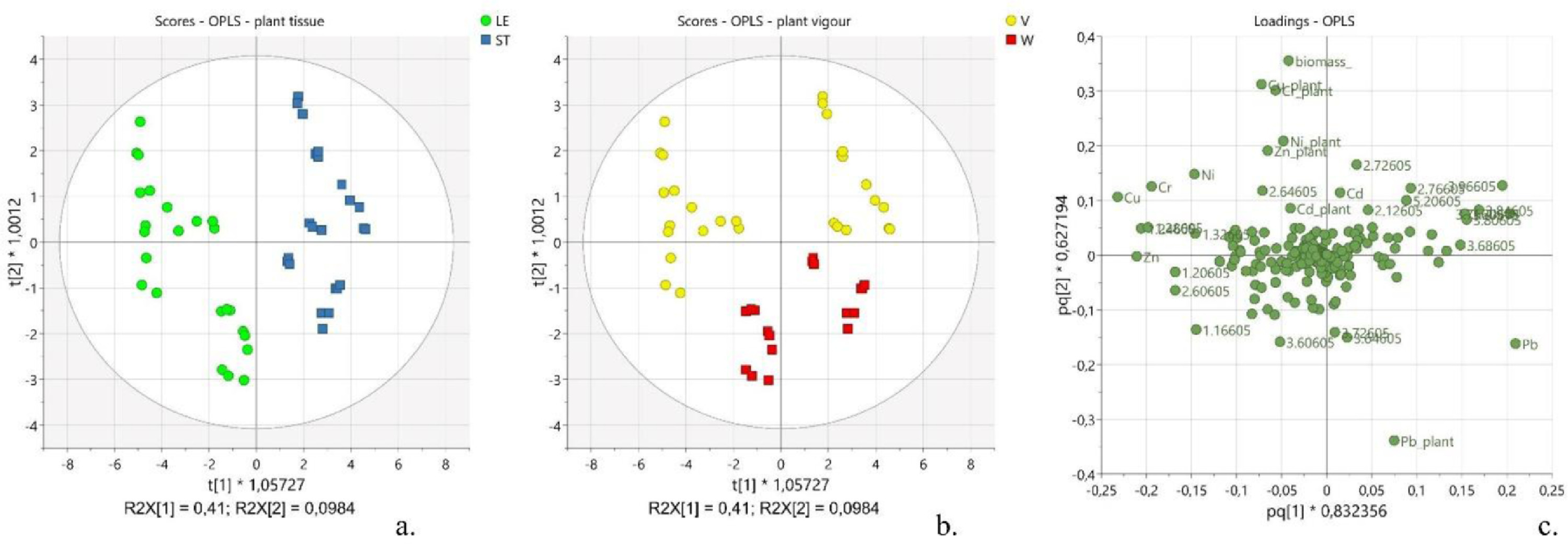
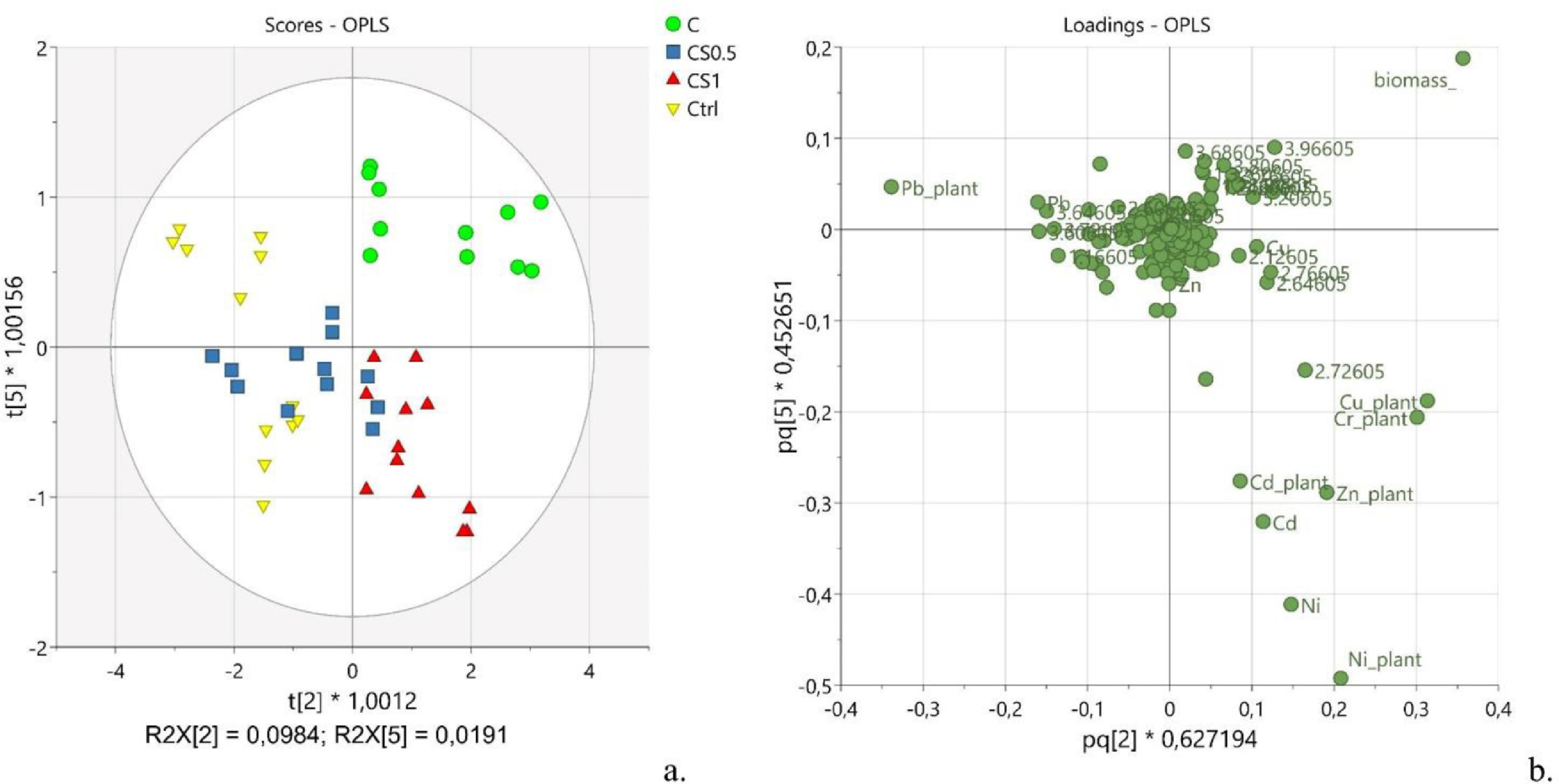
As a first task, the relation between the variations in the metabolic composition and the calculated amounts of residual heavy metals in the plant was considered. Thus, the OPLS analysis was performed considering the spectral buckets as x-variables, and the metal content and plant biomass as y-variables (see Supplementary materials for OPLS model parameters, Table S4). Such a model was characterized by five predictive components P1-P5 that explained 59.5% of x-variance (R2X[cum] = 0.595), 92.2% of y-variance (R2Y[cum] = 0.922) and 87.6% of y-variance modelled by x-variables (R2 = 0.876), and eight orthogonal components O1-O8 that explained about 38% of the x-variance unrelated to y-variance (R2X[cum] = 0.383). Two main separate clusters of observations were noticeable along P1. They corresponded to the two parts of the plant, namely the leaves (LE) and the stems (ST) (Fig. 2a, LE vs. ST). Inside each group, two evident subgroups were distinguishable along P2, which were identified as the vigorous plants (V) and the weak ones (W) (Fig. 2b, V vs. W). The components P1 and P2 explained 41 and 9.8% of the information in x-space (R2X[P1] = 0.41 and R2X[P2] = 0.098), suggesting an important variability of the metabolic composition along with the different tissues of the plant and according to the wellness of the plant. Indeed, the same components P1 and P2 explained 48.5 and 15.2% of the information in y-space (R2Y[P1] = 0.485 and R2Y[P2] = 0.152), suggesting a differential distribution of the y-variables, namely the metals concentration and the plant biomass, according to the different tissue of the plant and the health status of the plant (Fig. 2c). The visual inspection of the original harvested plants (see Supplementary materials for further details, Fig. S1), combined with the plant biomass values, suggested that the samples included in the subgroup with t[2]<0 (Fig. 2b, red scores) corresponded to the less healthy plants (weak, W), whereas the observations included in the subgroup with t[2]>0 corresponded to the more vigorous plants (V, Fig. 2b, yellow scores). The analysis of the loading plot (pq[1] vs. pq[2]) allowed the identification of the most significant variables towards the distribution of the scores, including the metal concentration, biomass amount, and spectral regions (Fig. 2c).
|
The amounts of Cu, Cr, Zn, and Ni added during the present study were well tolerated by the plants since the cluster containing the vigorous plants was characterized by having higher levels of them. On the other side, a lower tolerance was observed towards Pb as the highest levels of this metal were found in the weakest plants (Fig. 2c). It is well documented that the tolerance of hemp towards heavy metals is highly selective[26], and it is related both to the ability of the plant to produce high levels of antioxidant agents and to accumulate metals in roots, hindering them to translocate to the upper parts of the plant.[52][53] A selective accumulation of the studied metals in the leaves was observed according to the following trend: Cu > Zn > Cr > Ni > Cd >> Pb. Specifically, the observed accumulation of copper inside the leaves compared to the stems (Fig. 2c, pq[1] < -0.2), and, particularly, in leaves collected from vigorous green plants (Fig. 2c, pq[2] > 0.1), can be justified by the fact that this metal is actively involved in cellular functions happening mostly in this part of the plant, such as the transport of electrons in mitochondria and chloroplasts, the control of the cellular redox state, and the remodeling of the cell wall.[54]
The accumulation of zinc inside the leaves (Fig. 2c, pq[1] < -0.2) can be explained with both the increased production of organic acid and the vacuolar compartmentalization occurring in the leaves.[55]
While cadmium did not show any preferential site accumulation (Fig. 2c, pq[1] ≈ 0), lead presented a noticeable preference to distribute within the stems (Fig. 2c, pq[1] > 0.2). Interestingly, the tolerance of hemp towards high levels of lead was lower compared to the other selected heavy metals. Such evidence is not surprising considering the high toxicity of lead on living organisms.[56] Indeed, lead impairs the physicochemical properties of soil and soil microbial community; it alters the water and nutrient uptake, generates reactive oxygen species (ROS), and reduces photosynthetic activity, hindering plant growth and development.[55] As a result of the present investigation, the residual amounts of lead were found higher in the less healthy plants (Fig. 2c, pq[1] < -0.1). Also, this evidence is confirmed by the inverse correlation observed in the loading plot between the plant biomass (Fig. 2c, pq[2] > 0.3) and the level of lead in the plant (Fig. 2c, pq[2] < -0.3), suggesting a potential phytotoxic effect of lead.
The analysis of the correlation matrix (see Supplementary materials for further details, Table S5) related to the OPLS model helped to better understand the ability of hemp to uptake simultaneously and selectively the six metals under investigation. A positive correlation was found for the plant content of Cu with Zn (r = 0.80) and Ni (r = 0.82). Also, Zn correlated well with the plant content of Cd (r = 0.84) and Ni (r = 0.81). Conversely, a negative correlation was found between Cu plant content and Pb residual amount (r = -0.82). Such evidence may suggest a competition between lead and copper during the uptake stage. Importantly, it was found that the plant weight (biomass) positively correlated with the residual content of Cu (r = 0.72) and Cr (r = 0.64) in the plant. Still, a negative correlation was found between the plant biomass and the Pb content (r = -0.76), supporting the hypothesis that hemp tolerance towards this metal is relatively low compared to the other metals investigated in the present work.
Metabolic analysis of leaves and stems
The variations in the metabolic composition of the aqueous extracts of leaves and stems were investigated by performing an orthogonal partial least squares-discriminant analysis (OPLS-DA). A clear separation between the two classes of samples (leaves vs. stems) along the first two components ((R2X[1] = 0.394, R2Xo[1] = 0.27; see Supplementary materials for the scores plot, Fig. S2a) was obtained and the analysis of the S-plot allowed for the detection of the most significant x-variables and, thus, the metabolites contributing to such clustering (see Supplementary materials for the S-plot, Fig. S2b). Specifically, the buckets having values of VIPpredictive >1 and values of p(corr)1 >|0.5| were analyzed more deeply (see Supplementary materials for further details, Table S6).
The spectral regions containing signals related to glucose, fructose, and galactose moieties contributed significantly to the clustering of the stems. Also, the buckets containing the signals assigned to the organic acids were characterized by significant values of VIP and p(corr)1. An analogous trend was observed for the buckets related to the signals assigned to γ-aminobutyric acid (GABA) at 1.89–1.93 ppm and 3.01–3.05 ppm, and glutamine at 2.13–2.17 ppm.
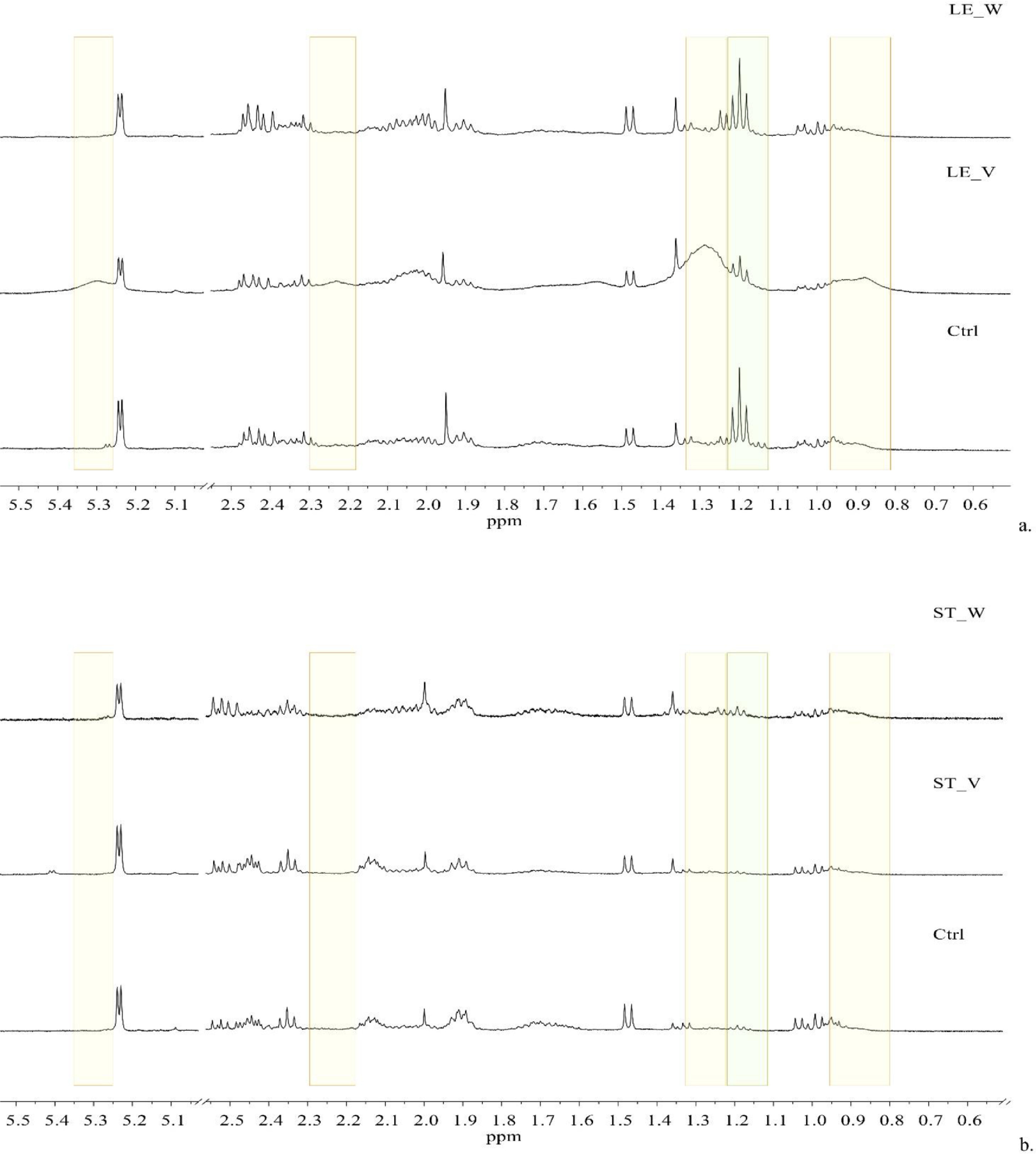
Interestingly, the clustering of the leaves was affected by some regions of the spectra containing very broad signals at 0.88, 1.29, 2.23, and 5.31 ppm (Fig. 3a, yellow rectangles), presumably attributable to the lipids of the cell membranes and/or walls.[57] The comparison of the 1D 1H NOESY spectra of the leaves collected from control plants (Ctrl with no metal contamination, Fig. 3a), vigorous plants (LE_V, Fig. 3a), and weak plants (LE_W, Fig. 3a) indicated that such broad signals were predominant in vigorous plants. Such broad signals were not observed in the aqueous extracts of stems (Fig. 3b). Also, the buckets containing the signals of ethanol at 1.17–1.25 ppm contributed relevantly to the grouping of the leaves towards the stems (Fig. 3a and b, green rectangular). These data could be explained by the fact that the leaves and, particularly the leaves sampled from vigorous green plants, have active photosynthetic processes and intact cell structures, whereas stems of weakest plants begin to enrich with lignin and have less active photosynthesis.[58][59]
|
The spectral regions containing these broad signals correlated positively with the calculated amounts of residual copper in the leaves. Under physiological conditions, Cu is found in two common forms: Cu(I) (low oxidation state) preferentially binding sulfur-containing compounds having a thiol or a thioether group, and Cu(II) (high oxidation state), that coordinates mainly with oxygen or imidazole nitrogen groups. The main functions of Cu are the transport of electrons in mitochondria and chloroplasts, the control of the cellular redox state (being a major Cu-binding protein the Cu/Zn superoxide dismutase), and the remodeling of the cell wall, which is one of the major Cu-accumulation sites in hyper-accumulating plants.[54] Moreover, Cu leaf content is inversely associated with the intensity of the buckets relating to glucose and fructose (in the range [3.26–4.08] ppm) and ethanol (centered at 1.18 and 3.62 ppm). In weakest plants, sucrose, the main soluble component of the phloem sap and translatable product of photosynthesis might be hydrolyzed into the two constituting monomers (glucose and fructose) and fermented by yeast and/or bacteria producing ethanol.[60]
Effect of treatment with spirulina
In a second task, the effect of the treatment with spirulina during the cultivation of hemp towards the remediation of contaminated soil was investigated. Thus, a deeper look was taken at the higher components of the OPLS model described in the prior subsection. Indeed, a good clustering of samples according to the treatment was visible in the t[2] vs. t[5] scores plot (Fig. 4a). The second component and the fifth one explained together 11.75% of the x-variance predictive of the y-variance (R2X[P2] = 0.098 and R2X[P5] = 0.019), suggesting that the treatment with spirulina affected slightly the metabolic composition of the plant. Such evidence is relevant to make possible a re-utilization of the hemp, which does not change its metabolome during the phytoremediation stage.
|
Specifically, the samples collected from plants grown in contaminated soil (Fig. 4a, green circles, C) and the samples obtained from plants treated with a higher concentration of spirulina (Fig. 4a, red triangles, CS1) tended to cluster at values of t[2] >0. Conversely, the samples derived from plants treated with a lower concentration of the blue-green alga (Fig. 4a, blue squares, CS0.5) and the control ones (Fig. 4a, yellow triangles, Ctrl) were distributed preferentially at values of t[2] <0. A further piece of evidence was that samples C and the samples CS1 tended to separate into two clear groups along t[5]. Analysis of the loading plot (Fig. 4b) suggested that the uptake of all the metals, except for lead, contributed relevantly to the clustering of the plants treated with a higher dose of spirulina (CS1). Also, the average plant biomass (pq[2] > 0.35) was generally higher in contaminated plants compared to the uncontaminated ones. In addition, the plants that grew in contaminated soil and were irrigated with a higher dose of spirulina (CS1) presented a higher value of biomass, confirming the activity of these cyanobacteria to promote plant growth. The average plant contents of Ni (pq[2] = 0.21), Zn (pq[2] = 0.19), and Cd (pq[2] = 0.09) were greater in contaminated plants grown in soils added with spirulina than those grown without it.
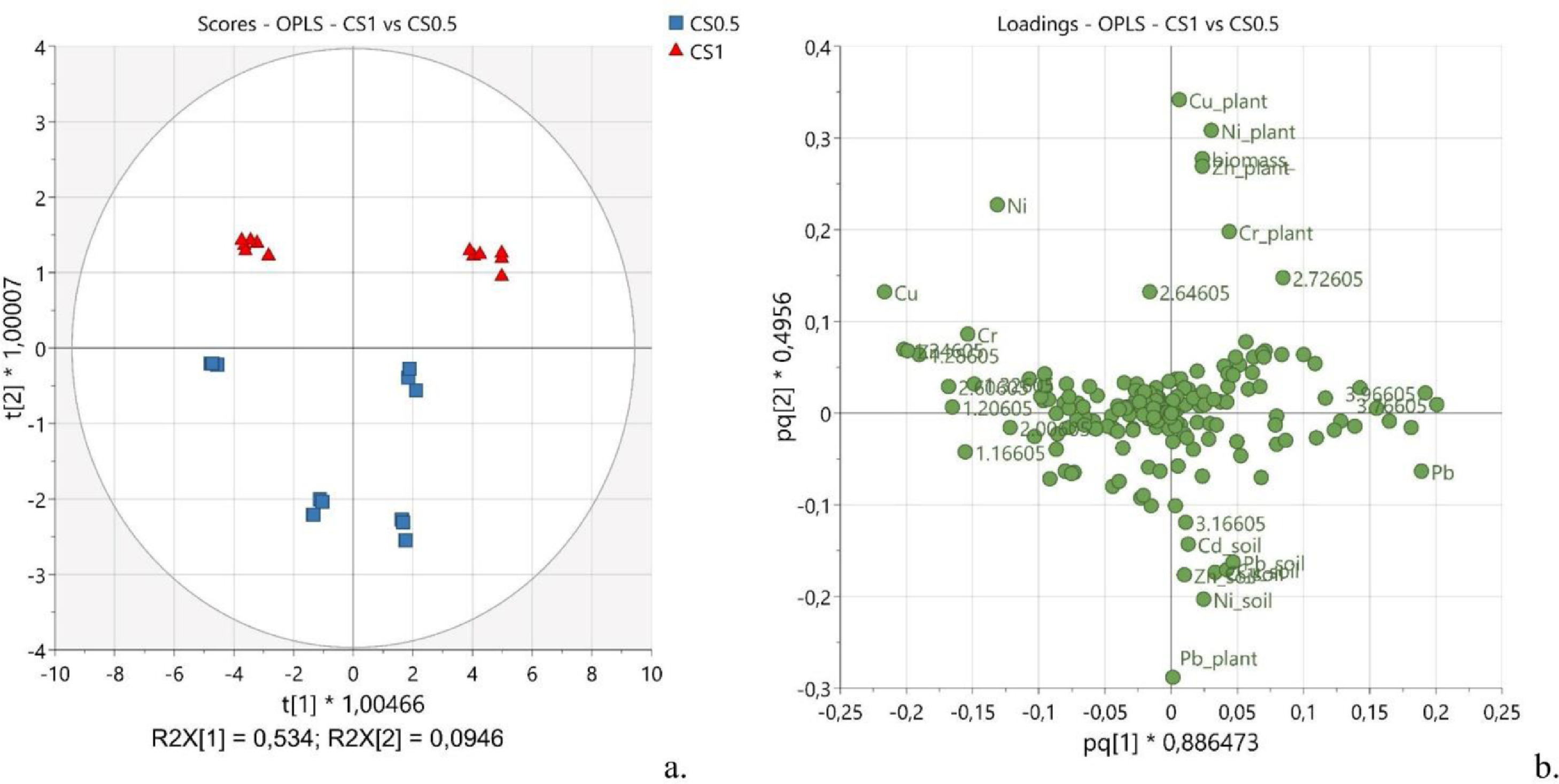
To get deeper insights into the effects deriving from the dose of spirulina, OPLS was applied to CS1 and CS0.5 samples. The observations, namely CS1 (Fig. 5a, red triangles) and CS0.5 (Fig. 5a, blue squares), separated along the second predictive component P2, explaining about 9.5% of the predictive x-variance (R2X[P2] = 0.095). The distribution was strongly affected by the part of the plant the samples derived from, leaves or stems, with a noticeable clustering along the first predictive component P1 (R2X[P1] = 0.534). The corresponding loading plot (Fig. 5b) showed important dose-dependent variations. Indeed, nickel, zinc, copper, and chromium were more abundant in samples treated with a higher dose of spirulina (CS1).
|
Generally, as the concentration of spirulina increased, the residual metal content increased both in the leaves and in the stems (for Cu and Zn, the increase was more noticeable in stems than in leaves), whereas the Cr content increased in leaves and decreased in stems. While no dose-effect was observed for Cd, noticeable variations were exerted by increased amounts of spirulina on the quantities of Pb incorporated into the plant. Specifically, the average content of Pb in the plant was higher in CS0.5 than in CS1, suggesting that a higher amount of the blue-green alga contained in the soil might compete with hemp to sequester this metal.
A piece of further evidence was that the average soil residual of all heavy metals resulted higher when it was irrigated with a higher concentration of spirulina (CS0.5 vs CS1), suggesting that the cyanobacteria stationing in the soil may exert a strong action of metal chelation, avoiding leaching of them during the watering stage and acting as a reservoir for hemp to absorb the chelated metals. Such results find fundamentals in the reported ability of Arthrospira platensis to adsorb (in form of dry biomass) and accumulate (in form of alive biomass) all the six metals here tested through various mechanisms (ion exchange, binding to functional groups, complexation, and microprecipitation).[45]
Very small effects were observed on the metabolic profile when samples CS1 were compared with CS0.5. Interestingly, according to the loading plot (Fig. 5b), the buckets at 2.66 and 2.74 ppm, containing the signals assigned to citric acid, contributed importantly to the observed grouping between the samples treated with the two different doses of spirulina (see Supplementary materials for the variable trend, Fig. S3). Specifically, the signals of citric acid were more intense in samples treated with a higher dose of spirulina. Considering that citric acid is one of the main chelating agents in plants, this evidence may support the hypothesis that, at a higher concentration, the cyanobacteria can chelate the heavy metals sequestering them into the soil. As a result, less quantity of heavy metal is available for the plant to uptake, and, thus, more chelating agents inside the plant are in the free form not coordinating any metal.[61]
Conclusions
It was demonstrated that hemp accumulates copper, chromium, nickel, and zinc preferentially in the leaves, while lead is distributed mainly in the stems of the plant. Such selective compartmentalization is enhanced when the plant is irrigated with water containing spirulina. It was found that, at higher concentrations, spirulina acts as a growth promoter, contributing to an increase in the final generated biomass. Also, it was demonstrated that the treatment with spirulina during the cultivation of the hemp induced an enhancement in the uptake of heavy metals, except for lead. Such a result may be explained by assuming a strong affinity of spirulina towards the lead, resulting in confinement of this metal inside the soil and hampering its uptake by the plant. The NMR analysis allowed to identify the crucial variations of the metabolic composition that were induced by the treatment with spirulina. Results reported in this work pave the way to further studies aimed to understand the optimal dose of spirulina to enhance the efficiency of this innovative combined remediation bio-system. Furthermore, the results described may encourage the application of spectroscopic methods for the rapid detection of structural changes in the various environmental spheres, allowing prompt intervention through the adoption of remediation schemes.
Supplementary materials
- Supporting information (.docx)
Acknowledgements
The authors acknowledge Dr. Vito Bruno, Dr. Francesca Portincasa, and Dr. Stefano Todisco for the helpful discussions and technical support.
Funding
This study was funded by Regione Puglia “Sezione Competitività delle filiere agroalimentari” under the call “Avviso pubblico per la presentazione di progetti di ricerca ed innovazione e interventi a carattere pilota con D.D. 189 del 17/10/2018 - Project “BIO.SP.HE.RE - Bio-integrated SPirulina and HEmp REmediation”.
Conflict of interest
None stated.
References
- ↑ 1.0 1.1 1.2 Awa, Soo Hui; Hadibarata, Tony (1 February 2020). "Removal of Heavy Metals in Contaminated Soil by Phytoremediation Mechanism: a Review" (in en). Water, Air, & Soil Pollution 231 (2): 47. doi:10.1007/s11270-020-4426-0. ISSN 0049-6979. http://link.springer.com/10.1007/s11270-020-4426-0.
- ↑ Vareda, João P.; Valente, Artur J.M.; Durães, Luisa (1 September 2019). "Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review" (in en). Journal of Environmental Management 246: 101–118. doi:10.1016/j.jenvman.2019.05.126. https://linkinghub.elsevier.com/retrieve/pii/S0301479719307546.
- ↑ Arora, Monu; Kiran, Bala; Rani, Shweta; Rani, Anchal; Kaur, Barinder; Mittal, Neeraj (15 December 2008). "Heavy metal accumulation in vegetables irrigated with water from different sources" (in en). Food Chemistry 111 (4): 811–815. doi:10.1016/j.foodchem.2008.04.049. https://linkinghub.elsevier.com/retrieve/pii/S0308814608005013.
- ↑ Kumar, Sandeep; Prasad, Shiv; Yadav, Krishna Kumar; Shrivastava, Manoj; Gupta, Neha; Nagar, Shivani; Bach, Quang-Vu; Kamyab, Hesam et al. (1 December 2019). "Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches - A review" (in en). Environmental Research 179: 108792. doi:10.1016/j.envres.2019.108792. https://linkinghub.elsevier.com/retrieve/pii/S0013935119305894.
- ↑ Manzoor, Javid; Sharma, Manoj; Wani, Khursheed Ahmad (9 August 2018). "Heavy metals in vegetables and their impact on the nutrient quality of vegetables: A review" (in en). Journal of Plant Nutrition 41 (13): 1744–1763. doi:10.1080/01904167.2018.1462382. ISSN 0190-4167. https://www.tandfonline.com/doi/full/10.1080/01904167.2018.1462382.
- ↑ 6.0 6.1 Edelstein, Menahem; Ben-Hur, Meni (1 April 2018). "Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in horticultural crops" (in en). Scientia Horticulturae 234: 431–444. doi:10.1016/j.scienta.2017.12.039. https://linkinghub.elsevier.com/retrieve/pii/S0304423817307628.
- ↑ Rizvi, Asfa; Zaidi, Almas; Ameen, Fuad; Ahmed, Bilal; AlKahtani, Muneera D. F.; Khan, Mohd. Saghir (2020). "Heavy metal induced stress on wheat: phytotoxicity and microbiological management" (in en). RSC Advances 10 (63): 38379–38403. doi:10.1039/D0RA05610C. ISSN 2046-2069. PMC PMC9121104. PMID 35693041. http://xlink.rsc.org/?DOI=D0RA05610C.
- ↑ Tiwari, Shalini; Lata, Charu (6 April 2018). "Heavy Metal Stress, Signaling, and Tolerance Due to Plant-Associated Microbes: An Overview". Frontiers in Plant Science 9: 452. doi:10.3389/fpls.2018.00452. ISSN 1664-462X. PMC PMC5897519. PMID 29681916. http://journal.frontiersin.org/article/10.3389/fpls.2018.00452/full.
- ↑ Ali, Hazrat; Khan, Ezzat (18 August 2019). "Trophic transfer, bioaccumulation, and biomagnification of non-essential hazardous heavy metals and metalloids in food chains/webs—Concepts and implications for wildlife and human health" (in en). Human and Ecological Risk Assessment: An International Journal 25 (6): 1353–1376. doi:10.1080/10807039.2018.1469398. ISSN 1080-7039. https://www.tandfonline.com/doi/full/10.1080/10807039.2018.1469398.
- ↑ Pratush, Amit; Kumar, Ajay; Hu, Zhong (1 September 2018). "Adverse effect of heavy metals (As, Pb, Hg, and Cr) on health and their bioremediation strategies: a review" (in en). International Microbiology 21 (3): 97–106. doi:10.1007/s10123-018-0012-3. ISSN 1139-6709. http://link.springer.com/10.1007/s10123-018-0012-3.
- ↑ Rehman, Kanwal; Fatima, Fiza; Waheed, Iqra; Akash, Muhammad Sajid Hamid (1 January 2018). "Prevalence of exposure of heavy metals and their impact on health consequences" (in en). Journal of Cellular Biochemistry 119 (1): 157–184. doi:10.1002/jcb.26234. ISSN 0730-2312. https://onlinelibrary.wiley.com/doi/10.1002/jcb.26234.
- ↑ Olsson, Per-Erik; Kling, Peter; Hogstrand, Christer (1998), Langston, William J.; Bebianno, Maria João, eds., "Mechanisms of heavy metal accumulation and toxicity in fish" (in en), Metal Metabolism in Aquatic Environments (Boston, MA: Springer US): 321–350, doi:10.1007/978-1-4757-2761-6_10, ISBN 978-1-4419-4731-4, http://link.springer.com/10.1007/978-1-4757-2761-6_10. Retrieved 2022-09-11
- ↑ Tchounwou, Paul B.; Yedjou, Clement G.; Patlolla, Anita K.; Sutton, Dwayne J. (2012), Luch, Andreas, ed., "Heavy Metal Toxicity and the Environment" (in en), Molecular, Clinical and Environmental Toxicology (Basel: Springer Basel) 101: 133–164, doi:10.1007/978-3-7643-8340-4_6, ISBN 978-3-7643-8339-8, PMC PMC4144270, PMID 22945569, http://link.springer.com/10.1007/978-3-7643-8340-4_6. Retrieved 2022-09-11
- ↑ Zwolak, Aneta; Sarzyńska, Magdalena; Szpyrka, Ewa; Stawarczyk, Kinga (1 July 2019). "Sources of Soil Pollution by Heavy Metals and Their Accumulation in Vegetables: a Review" (in en). Water, Air, & Soil Pollution 230 (7): 164. doi:10.1007/s11270-019-4221-y. ISSN 0049-6979. http://link.springer.com/10.1007/s11270-019-4221-y.
- ↑ Vardhan, Kilaru Harsha; Kumar, Ponnusamy Senthil; Panda, Rames C. (1 September 2019). "A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives" (in en). Journal of Molecular Liquids 290: 111197. doi:10.1016/j.molliq.2019.111197. https://linkinghub.elsevier.com/retrieve/pii/S0167732219317684.
- ↑ Wuana, Raymond A.; Okieimen, Felix E. (24 October 2011). "Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation" (in en). ISRN Ecology 2011: 1–20. doi:10.5402/2011/402647. ISSN 2090-4614. https://www.hindawi.com/journals/isrn/2011/402647/.
- ↑ Dhaliwal, Salwinder Singh; Singh, Jaswinder; Taneja, Parminder Kaur; Mandal, Agniva (1 January 2020). "Remediation techniques for removal of heavy metals from the soil contaminated through different sources: a review" (in en). Environmental Science and Pollution Research 27 (2): 1319–1333. doi:10.1007/s11356-019-06967-1. ISSN 0944-1344. http://link.springer.com/10.1007/s11356-019-06967-1.
- ↑ Gong, Yanyan; Zhao, Dongye; Wang, Qilin (1 December 2018). "An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade" (in en). Water Research 147: 440–460. doi:10.1016/j.watres.2018.10.024. https://linkinghub.elsevier.com/retrieve/pii/S0043135418308212.
- ↑ Gusiatin, Zygmunt M.; Kulikowska, Dorota; Klik, Barbara (27 August 2020). "New-Generation Washing Agents in Remediation of Metal-Polluted Soils and Methods for Washing Effluent Treatment: A Review" (in en). International Journal of Environmental Research and Public Health 17 (17): 6220. doi:10.3390/ijerph17176220. ISSN 1660-4601. PMC PMC7503436. PMID 32867145. https://www.mdpi.com/1660-4601/17/17/6220.
- ↑ Qin, Huaqing; Hu, Tianjue; Zhai, Yunbo; Lu, Ningqin; Aliyeva, Jamila (1 March 2020). "The improved methods of heavy metals removal by biosorbents: A review" (in en). Environmental Pollution 258: 113777. doi:10.1016/j.envpol.2019.113777. https://linkinghub.elsevier.com/retrieve/pii/S0269749119351218.
- ↑ Eid, Ebrahem M.; Shaltout, Kamal H. (1 November 2016). "Bioaccumulation and translocation of heavy metals by nine native plant species grown at a sewage sludge dump site" (in en). International Journal of Phytoremediation 18 (11): 1075–1085. doi:10.1080/15226514.2016.1183578. ISSN 1522-6514. https://www.tandfonline.com/doi/full/10.1080/15226514.2016.1183578.
- ↑ Hernández-Allica, Javier; Becerril, José M.; Garbisu, Carlos (1 March 2008). "Assessment of the phytoextraction potential of high biomass crop plants" (in en). Environmental Pollution 152 (1): 32–40. doi:10.1016/j.envpol.2007.06.002. https://linkinghub.elsevier.com/retrieve/pii/S0269749107002722.
- ↑ Pachura, Piotr; Ociepa-Kubicka, Agnieszka; Skowron-Grabowska, Beata (14 January 2016). "Assessment of the availability of heavy metals to plants based on the translocation index and the bioaccumulation factor" (in en). Desalination and Water Treatment 57 (3): 1469–1477. doi:10.1080/19443994.2015.1017330. ISSN 1944-3994. http://www.tandfonline.com/doi/full/10.1080/19443994.2015.1017330.
- ↑ Ahmad, Rafiq; Tehsin, Zara; Malik, Samina Tanvir; Asad, Saeed Ahmad; Shahzad, Muhammad; Bilal, Muhammad; Shah, Mohammad Maroof; Khan, Sabaz Ali (1 February 2016). "Phytoremediation Potential of Hemp ( Cannabis sativa L.): Identification and Characterization of Heavy Metals Responsive Genes: Biotechnology" (in en). CLEAN - Soil, Air, Water 44 (2): 195–201. doi:10.1002/clen.201500117. https://onlinelibrary.wiley.com/doi/10.1002/clen.201500117.
- ↑ Morin-Crini, Nadia; Loiacono, Sonia; Placet, Vincent; Torri, Giangiacomo; Bradu, Corina; Kostić, Mirjana; Cosentino, Cesare; Chanet, Gilles et al. (1 March 2019). "Hemp-based adsorbents for sequestration of metals: a review" (in en). Environmental Chemistry Letters 17 (1): 393–408. doi:10.1007/s10311-018-0812-x. ISSN 1610-3653. http://link.springer.com/10.1007/s10311-018-0812-x.
- ↑ 26.0 26.1 26.2 Zielonka, Dariusz; Szulc, Wiesław; Skowrońska, Monika; Rutkowska, Beata; Russel, Stefan (25 June 2020). "Hemp-Based Phytoaccumulation of Heavy Metals from Municipal Sewage Sludge and Phosphogypsum Under Field Conditions" (in en). Agronomy 10 (6): 907. doi:10.3390/agronomy10060907. ISSN 2073-4395. https://www.mdpi.com/2073-4395/10/6/907.
- ↑ Adesina, Ifeoluwa; Bhowmik, Arnab; Sharma, Harmandeep; Shahbazi, Abolghasem (14 April 2020). "A Review on the Current State of Knowledge of Growing Conditions, Agronomic Soil Health Practices and Utilities of Hemp in the United States" (in en). Agriculture 10 (4): 129. doi:10.3390/agriculture10040129. ISSN 2077-0472. https://www.mdpi.com/2077-0472/10/4/129.
- ↑ Crini, Grégorio; Lichtfouse, Eric; Chanet, Gilles; Morin-Crini, Nadia (1 September 2020). "Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: a review" (in en). Environmental Chemistry Letters 18 (5): 1451–1476. doi:10.1007/s10311-020-01029-2. ISSN 1610-3653. https://link.springer.com/10.1007/s10311-020-01029-2.
- ↑ Schluttenhofer, Craig; Yuan, Ling (1 November 2017). "Challenges towards Revitalizing Hemp: A Multifaceted Crop" (in en). Trends in Plant Science 22 (11): 917–929. doi:10.1016/j.tplants.2017.08.004. https://linkinghub.elsevier.com/retrieve/pii/S1360138517301772.
- ↑ Rupasinghe, H. P. Vasantha; Davis, Amy; Kumar, Shanthanu K.; Murray, Beth; Zheljazkov, Valtcho D. (7 September 2020). "Industrial Hemp (Cannabis sativa subsp. sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals" (in en). Molecules 25 (18): 4078. doi:10.3390/molecules25184078. ISSN 1420-3049. PMC PMC7571072. PMID 32906622. https://www.mdpi.com/1420-3049/25/18/4078.
- ↑ Citterio, Sandra; Santagostino, Angela; Fumagalli, Pietro; Prato, Nadia; Ranalli, Paolo; Sgorbati, Sergio (2003). "Heavy metal tolerance and accumulation of Cd, Cr and Ni by Cannabis sativa L". Plant and Soil 256 (2): 243–252. doi:10.1023/A:1026113905129. http://link.springer.com/10.1023/A:1026113905129.
- ↑ Ćaćić, Marija; Perčin, Aleksandra; Zgorelec, Željka; Kisić, Ivica (2019). "Evaluation of heavy metals accumulation potential of hemp (Cannabis sativa L.)" (in en). Journal of Central European Agriculture 20 (2): 700–711. doi:10.5513/JCEA01/20.2.2201. ISSN 1332-9049. https://jcea.agr.hr/en/issues/article/2201.
- ↑ Piotrowska-Cyplik, A.; Czarnecki, Z. (2003). "Phytoextraction of Heavy Metals by Hemp during Anaerobic Sewage Sludge Management in the Non-Industrial Sites". Polish Journal of Environmental Studies 12 (6): 779–84. http://www.pjoes.com/Phytoextraction-of-Heavy-Metals-by-Hemp-r-nduring-Anaerobic-Sewage-Sludge-Management,87620,0,2.html.
- ↑ Bosecker, Klaus (1 July 1997). "Bioleaching: metal solubilization by microorganisms" (in en). FEMS Microbiology Reviews 20 (3-4): 591–604. doi:10.1111/j.1574-6976.1997.tb00340.x. ISSN 1574-6976. https://academic.oup.com/femsre/article-lookup/doi/10.1111/j.1574-6976.1997.tb00340.x.
- ↑ Drobíková, Klára; Rozumová, Lucia; Otoupalíková, Hana; Seidlerová, Jana (1 January 2015). "Bioleaching of hazardous waste". Chemical Papers 69 (9). doi:10.1515/chempap-2015-0121. ISSN 1336-9075. https://www.degruyter.com/document/doi/10.1515/chempap-2015-0121/html.
- ↑ Mishra, Debaraj; Kim, Dong-Jin; Ahn, Jong-Gwan; Rhee, Young-Ha (1 June 2005). "Bioleaching: A microbial process of metal recovery; A review" (in en). Metals and Materials International 11 (3): 249–256. doi:10.1007/BF03027450. ISSN 1598-9623. http://link.springer.com/10.1007/BF03027450.
- ↑ Okoh, M.P.; Olobayetan, I.W.; Machunga-Mambula, S.S. (2018). "Bioleaching, a technology for metal extraction and remediation: Mitigating health consequences for metal exposure". International Journal of Development and Sustainability 7 (7): 2103-2118. https://isdsnet.com/ijds-v7n7.html.
- ↑ Rawlings, Douglas E. (1 October 2002). "Heavy Metal Mining Using Microbes" (in en). Annual Review of Microbiology 56 (1): 65–91. doi:10.1146/annurev.micro.56.012302.161052. ISSN 0066-4227. https://www.annualreviews.org/doi/10.1146/annurev.micro.56.012302.161052.
- ↑ Sun, Wenjie; Cheng, Kai; Sun, Kevin Y.; Ma, Xingmao (1 June 2021). "Microbially Mediated Remediation of Contaminated Sediments by Heavy Metals: a Critical Review" (in en). Current Pollution Reports 7 (2): 201–212. doi:10.1007/s40726-021-00175-7. ISSN 2198-6592. https://link.springer.com/10.1007/s40726-021-00175-7.
- ↑ Balaji, Sundaramoorthy; Kalaivani, Thiagarajan; Rajasekaran, Chandrasekaran (1 April 2014). "Biosorption of Zinc and Nickel and Its Effect on Growth of Different Spirulina Strains: Biosorption Potentials of Spirulina Strains" (in en). CLEAN - Soil, Air, Water 42 (4): 507–512. doi:10.1002/clen.201200340. https://onlinelibrary.wiley.com/doi/10.1002/clen.201200340.
- ↑ Bhattacharya, Sanjib (2020). "The Role of Spirulina (Arthrospira) in the Mitigation of Heavy-Metal Toxicity: An Appraisal" (in en). Journal of Environmental Pathology, Toxicology and Oncology 39 (2): 149–157. doi:10.1615/JEnvironPatholToxicolOncol.2020034375. ISSN 0731-8898. http://www.dl.begellhouse.com/journals/0ff459a57a4c08d0,2306a38266045594,4463148f55e256f9.html.
- ↑ Kőnig-Péter, Anikó; Kilár, Ferenc; Felinger, Attila; Pernyeszi, Tímea (2015). "Biosorption characteristics of Spirulina and Chlorella cells to accumulate heavy metals" (in en). Journal of the Serbian Chemical Society 80 (3): 407–419. doi:10.2298/JSC140321060P. ISSN 0352-5139. http://www.doiserbia.nb.rs/Article.aspx?ID=0352-51391400060K.
- ↑ Nalimova, A. A.; Popova, V. V.; Tsoglin, L. N.; Pronina, N. A. (1 March 2005). "The effects of copper and zinc on Spirulina platensis growth and heavy metal accumulation in its cells" (in en). Russian Journal of Plant Physiology 52 (2): 229–234. doi:10.1007/s11183-005-0035-4. ISSN 1021-4437. http://link.springer.com/10.1007/s11183-005-0035-4.
- ↑ Zinicovscaia, Inga; Cepoi, Liliana; Chiriac, Tatiana; Ana Culicov, Otilia; Frontasyeva, Marina; Pavlov, Sergey; Kirkesali, Elena; Akshintsev, Artem et al. (20 May 2016). "Spirulina platensis as biosorbent of chromium and nickel from industrial effluents" (in en). Desalination and Water Treatment 57 (24): 11103–11110. doi:10.1080/19443994.2015.1042061. ISSN 1944-3994. http://www.tandfonline.com/doi/full/10.1080/19443994.2015.1042061.
- ↑ 45.0 45.1 Zinicovscaia, Inga; Safonov, Alexey; Ostalkevich, Svetlana; Gundorina, Svetlana; Nekhoroshkov, Pavel; Grozdov, Dmitrii (6 December 2019). "Metal ions removal from different type of industrial effluents using Spirulina platensis biomass" (in en). International Journal of Phytoremediation 21 (14): 1442–1448. doi:10.1080/15226514.2019.1633264. ISSN 1522-6514. https://www.tandfonline.com/doi/full/10.1080/15226514.2019.1633264.
- ↑ Tripathi, R.D.; Dwivedi, S.; Shukla, M.K.; Mishra, S.; Srivastava, S.; Singh, R.; Rai, U.N.; Gupta, D.K. (1 February 2008). "Role of blue green algae biofertilizer in ameliorating the nitrogen demand and fly-ash stress to the growth and yield of rice (Oryza sativa L.) plants" (in en). Chemosphere 70 (10): 1919–1929. doi:10.1016/j.chemosphere.2007.07.038. https://linkinghub.elsevier.com/retrieve/pii/S0045653507009162.
- ↑ 47.0 47.1 Wuang, Shy Chyi; Khin, Mar Cho; Chua, Pei Qiang Danny; Luo, Yanpei Darren (1 April 2016). "Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers" (in en). Algal Research 15: 59–64. doi:10.1016/j.algal.2016.02.009. https://linkinghub.elsevier.com/retrieve/pii/S2211926416300480.
- ↑ Cepoi, Liliana; Zinicovscaia, Inga; Rudi, Ludmila; Chiriac, Tatiana; Miscu, Vera; Djur, Svetlana; Strelkova, Ludmila; Vergel, Konstantin et al. (1 January 2020). "Growth and heavy metals accumulation by Spirulina platensis biomass from multicomponent copper containing synthetic effluents during repeated cultivation cycles" (in en). Ecological Engineering 142: 105637. doi:10.1016/j.ecoleng.2019.105637. https://linkinghub.elsevier.com/retrieve/pii/S0925857419303611.
- ↑ Tsukihara, Tomitake; Fukuyama, Keiichi; Tahara, Hiromasa; Katsube, Yukiteru; Matsuura, Yoshiki; Tanaka, Nobuo; Kakudo, Masao; Wada, Keishiro et al. (1 December 1978). "X-Ray Analysis of Ferredoxin from Spirulina platensis" (in en). The Journal of Biochemistry 84 (6): 1645–1647. doi:10.1093/oxfordjournals.jbchem.a132293. ISSN 1756-2651. https://academic.oup.com/jb/article-lookup/doi/10.1093/oxfordjournals.jbchem.a132293.
- ↑ Şeker, Ayşegül; Shahwan, Talal; Eroğlu, Ahmet E.; Yılmaz, Sinan; Demirel, Zeliha; Dalay, Meltem Conk (1 June 2008). "Equilibrium, thermodynamic and kinetic studies for the biosorption of aqueous lead(II), cadmium(II) and nickel(II) ions on Spirulina platensis" (in en). Journal of Hazardous Materials 154 (1-3): 973–980. doi:10.1016/j.jhazmat.2007.11.007. https://linkinghub.elsevier.com/retrieve/pii/S0304389407016020.
- ↑ De Backer, Benjamin; Maebe, Kevin; Verstraete, Alain G.; Charlier, Corinne (1 July 2012). "Evolution of the Content of THC and Other Major Cannabinoids in Drug-Type Cannabis Cuttings and Seedlings During Growth of Plants*: EVOLUTION OF MAJOR CANNABINOIDS CONTENT DURING GROWTH OF PLANTS" (in en). Journal of Forensic Sciences 57 (4): 918–922. doi:10.1111/j.1556-4029.2012.02068.x. https://onlinelibrary.wiley.com/doi/10.1111/j.1556-4029.2012.02068.x.
- ↑ McPartland, John M.; McKernan, Kevin J. (2017), Chandra, Suman; Lata, Hemant; ElSohly, Mahmoud A., eds., "Contaminants of Concern in Cannabis: Microbes, Heavy Metals and Pesticides" (in en), Cannabis sativa L. - Botany and Biotechnology (Cham: Springer International Publishing): 457–474, doi:10.1007/978-3-319-54564-6_22, ISBN 978-3-319-54563-9, http://link.springer.com/10.1007/978-3-319-54564-6_22. Retrieved 2022-09-11
- ↑ Petrová, Š.; Benešová, D.; Soudek, P. et al. (2012). "Enhancement of metal(loid)s phytoextraction by Cannabis sativa L.". Journal of Food, Agriculture and Environment 10 (1): 631–41. doi:10.1234/4.2012.2735. https://www.wflpublisher.com/Abstract/2735.
- ↑ 54.0 54.1 Printz, Bruno; Lutts, Stanley; Hausman, Jean-Francois; Sergeant, Kjell (6 May 2016). "Copper Trafficking in Plants and Its Implication on Cell Wall Dynamics". Frontiers in Plant Science 7. doi:10.3389/fpls.2016.00601. ISSN 1664-462X. PMC PMC4859090. PMID 27200069. http://journal.frontiersin.org/Article/10.3389/fpls.2016.00601/abstract.
- ↑ 55.0 55.1 Broadley, Martin R.; White, Philip J.; Hammond, John P.; Zelko, Ivan; Lux, Alexander (1 March 2007). "Zinc in plants" (in en). New Phytologist 173 (4): 677–702. doi:10.1111/j.1469-8137.2007.01996.x. ISSN 0028-646X. https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8137.2007.01996.x.
- ↑ Agency for Toxic Substances and Disease Registry (17 January 2020). "The ATSDR 2019 Substance Priority List". Substance Priority List. Agency for Toxic Substances and Disease Registry. https://www.atsdr.cdc.gov/spl/index.html.
- ↑ Cruciani, Oscar; Mannina, Luisa; Sobolev, Anatoli; Cametti, Cesare; Segre, AnnaLaura (10 May 2006). "An Improved NMR Study of Liposomes Using 1-Palmitoyl-2-oleoyl-sn-glycero-3-phospatidylcholine as Model" (in en). Molecules 11 (5): 334–344. doi:10.3390/11050334. ISSN 1420-3049. PMC PMC6148493. PMID 17962765. http://www.mdpi.com/1420-3049/11/5/334.
- ↑ Brazel, Ailbhe J; Ó’Maoiléidigh, Diarmuid S (27 March 2019). "Photosynthetic activity of reproductive organs" (in en). Journal of Experimental Botany 70 (6): 1737–1754. doi:10.1093/jxb/erz033. ISSN 0022-0957. https://academic.oup.com/jxb/article/70/6/1737/5368212.
- ↑ H., Pfanz; G., Aschan; R., Langenfeld-Heyser; C., Wittmann; M., Loose (1 April 2002). "Ecology and ecophysiology of tree stems: corticular and wood photosynthesis". Naturwissenschaften 89 (4): 147–162. doi:10.1007/s00114-002-0309-z. ISSN 0028-1042. http://link.springer.com/10.1007/s00114-002-0309-z.
- ↑ Winter, H.; Huber, S. C. (1 January 2000). "Regulation of Sucrose Metabolism in Higher Plants: Localization and regulation of Activity of Key Enzymes" (in en). Critical Reviews in Plant Sciences 19 (1): 31–67. doi:10.1080/07352680091139178. ISSN 0735-2689. https://www.tandfonline.com/doi/full/10.1080/07352680091139178.
- ↑ Arru, Laura; Rognoni, Sara; Baroncini, Micaela; Bonatti, Piera Medeghini; Perata, Pierdomenico (1 January 2004). "Copper localization in Cannabis sativa L. grown in a copper-rich solution" (in en). Euphytica 140 (1-2): 33–38. doi:10.1007/s10681-004-4752-0. ISSN 0014-2336. https://link.springer.com/10.1007/s10681-004-4752-0.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. The original article lists references in alphabetical order; however, this version lists them in order of appearance, by design. Everything else remains true to the original article, per the "NoDerivatives" portion of the distribution license.