Difference between revisions of "Journal:The cannabinoid content of legal cannabis in Washington State varies systematically across testing facilities and popular consumer products"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 41: | Line 41: | ||
To investigate these concerns, we analyzed a large dataset from Washington State’s seed-to-sale traceability system. This dataset comprises hundreds of thousands of measurements of the principal cannabinoids in commercial cannabis, including tetrahydrocannabinol (THC) and cannabidiol (CBD). These measurements are available for commercial products tested across all state-licensed laboratories since 2014, which allowed us to assess the cannabinoid composition of commercial products between laboratories, over time, and across strains. | To investigate these concerns, we analyzed a large dataset from Washington State’s seed-to-sale traceability system. This dataset comprises hundreds of thousands of measurements of the principal cannabinoids in commercial cannabis, including tetrahydrocannabinol (THC) and cannabidiol (CBD). These measurements are available for commercial products tested across all state-licensed laboratories since 2014, which allowed us to assess the cannabinoid composition of commercial products between laboratories, over time, and across strains. | ||
==Results== | |||
===The basic chemotype landscape of commercial cannabis=== | |||
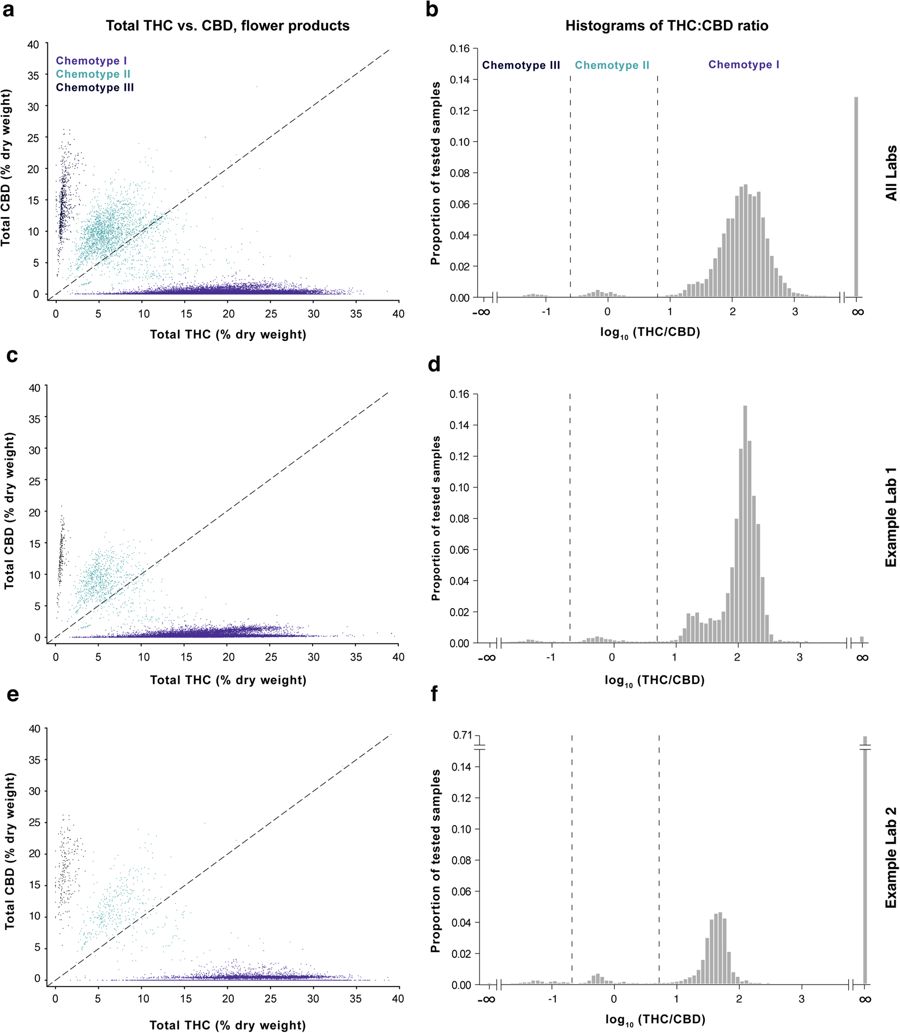
''Cannabis'' likely evolved in Central Asia, and [[Landrace|landraces]] native to regions including Afghanistan, Pakistan, India and China<ref name="HilligGenetic05">{{cite journal |title=Genetic evidence for speciation in ''Cannabis'' (Cannabaceae) |journal=Genetic Resources and Crop Evolution |author=Hillig, K.W. |volume=52 |issue=2 |pages=161–80 |year=2005 |doi=10.1007/s10722-003-4452-y}}</ref> have been found to fall into three general chemotypes based on genetically-constrained THC:CBD ratios.<ref name="deMeijerTheInher03">{{cite journal |title=The inheritance of chemical phenotype in ''Cannabis sativa'' L |journal=Genetics |author=de Meijer, E.P.; Bagatta, M.; Carboni, A. et al. |volume=163 |issue=1 |pages=335–46 |year=2003 |pmid=12586720 |pmc=PMC1462421}}</ref><ref name="HilligAChemo04">{{cite journal |title=A chemotaxonomic analysis of cannabinoid variation in ''Cannabis'' (Cannabaceae) |journal=American Journal of Botany |author=Hillig, K.W.; Mahlberg, P.G. |volume=91 |issue=6 |pages=966-75 |year=2004 |doi=10.3732/ajb.91.6.966 |pmid=21653452}}</ref> Consistent with previous work in landraces and commercial Dutch ''Cannabis''<ref name="HilligAChemo04" /><ref name="HazekampCannabis16">{{cite journal |title=Cannabis: From Cultivar to Chemovar II—A Metabolomics Approach to ''Cannabis'' Classification |journal=Cannabis and Cannabinoid Research |author=Hazekamp, A.; Tejkalová, K.; Papadimitriou, S. |volume=1 |issue=1 |year=2016 |doi=10.1089/can.2016.0017}}</ref>, we found that commercial ''Cannabis'' grown in Washington also conforms to this pattern (Fig. 1a–c). Unlike landraces, which are more likely to fall into the chemotype III (CBD-dominant) category and generally display lower overall levels of total THC<ref name="HilligAChemo04" />, most commercial ''Cannabis'' falls into the chemotype I category, characterized by relatively high total THC and low total CBD levels (Fig. 1d–f; see "Methods" section at the end for definition of total THC and CBD levels). While studying the chemotype landscape of these commercial samples, we observed striking differences in THC:CBD distributions across laboratories for both flower (Fig. 1d–f, Figure S1) and concentrates (Figure S2). This prompted us to examine interlab differences in more detail. In particular, we wished to assess whether this variation stemmed from intrinsic (e.g., methodological) differences between laboratories or from heterogeneity in the products submitted to those labs. | |||
[[File:Fig1 Jikomes SciReports2018 8.jpg|850px]] | |||
{{clear}} | |||
{| | |||
| STYLE="vertical-align:top;"| | |||
{| border="0" cellpadding="5" cellspacing="0" width="850px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Fig. 1''' The THC:CBD ratio defines three broad chemotypes of commercial cannabis flower measured by testing labs in Washington. Left column: Scatterplots of total THC vs. total CBD levels for cannabis flower. Right column: Histograms showing the THC:CBD ratio on a log scale and indicating the proportion of flower samples for each chemotype. Data are displayed for measurements batched across all Labs A-F (panels a-b; n = 175,136), for the lab reporting the lowest mean total THC levels (Lab A; panels c-d; n = 62,719), and the lab reporting the highest mean total THC levels (Lab F; panels e-f; n=26,664). Histograms for each of the six labs contributing to batched data in panels a-b are shown in Figure S1. Panels a and c were subsampled to n=50,000 for visualization purposes.</blockquote> | |||
|- | |||
|} | |||
|} | |||
===THC and CBD measurements vary widely across testing laboratories=== | |||
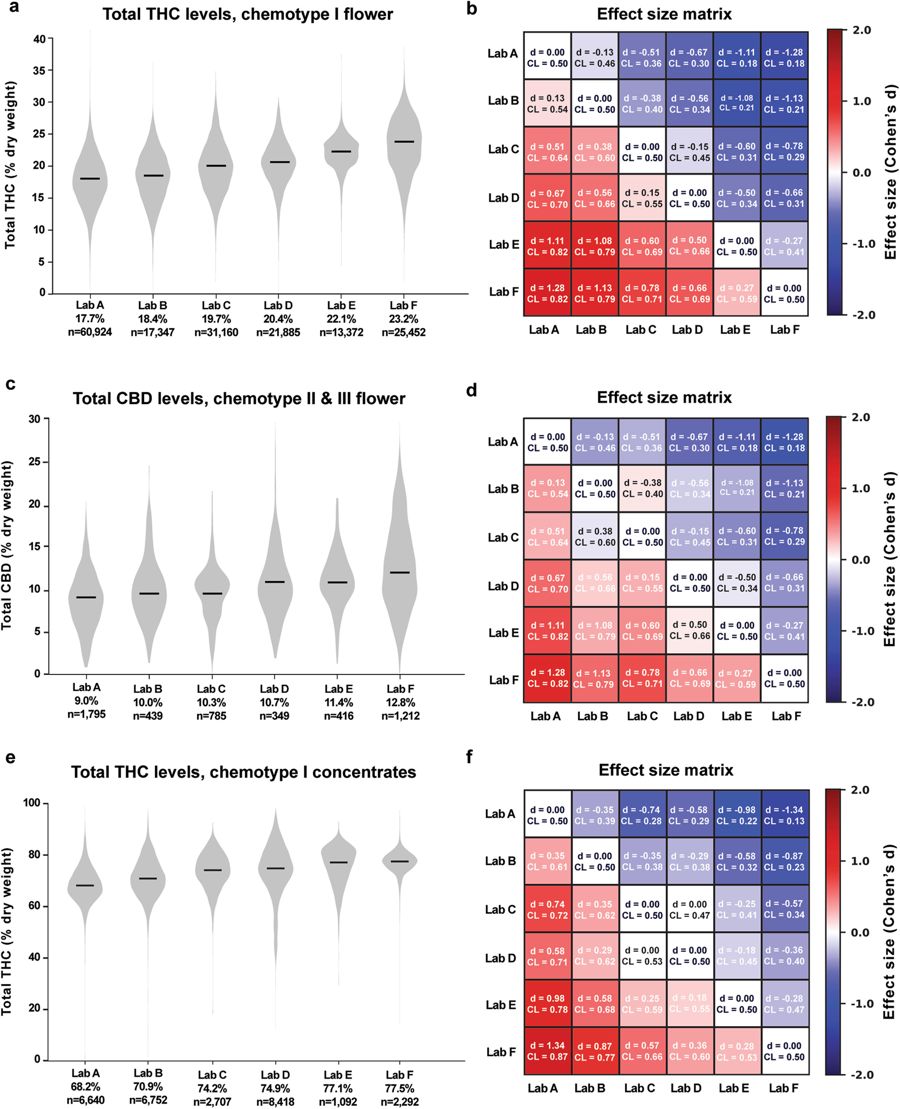
To compare cannabinoid measurements across labs, we looked at distributions of total THC and CBD levels for the six largest laboratories by data volume for different chemotypes and product categories. These labs, referred to henceforth as labs A-F, are Confidence Analytics (Lab A), Analytical 360 (Lab B), Green Grower Labs (Lab C), Integrity Labs (Lab D), Testing Technologies (Lab E), and Peak Analytics (Lab F). We observed differences in reported values of both THC and CBD (Fig. 2). For example, the median total THC content for chemotype I flower products ranged from 17.7% to 23.2% between the labs reporting the lowest and highest THC levels, respectively (Fig. 2a; labs A-F ordered from lowest to highest median reported THC levels). Pairwise differences in mean THC content between labs were statistically significant (p < 0.001 for each pairwise comparison in Fig. 2a, two-sided t-test). To quantify the magnitude of differences between labs, we calculated the effect sizes of pairwise differences using two metrics: Cohen’s d, the standardized difference between two means<ref name="CohenStatistical88">{{cite book |title=Statistical power analysis for the behavioral sciences |author=Cohen, J. |publisher=L. Eribaum Associates |edition=2nd |year=1988 |isbn=9780805802832}}</ref>, and a “Common Language” (CL) effect size, the probability that a random value from one sample will be greater than a random value from the other<ref name="McGrawAComm92">{{cite journal |title=A common language effect size statistic |journal=Psychological Bulletin |author=McGraw, K.O.; Wong, S.P. |volume=111 |issue=2 |pages=361–65 |year=1992 |doi=10.1037/0033-2909.111.2.361}}</ref> (Fig. 2b; see Analytical Methods). | |||
[[File:Fig2 Jikomes SciReports2018 8.jpg|850px]] | |||
{{clear}} | |||
{| | |||
| STYLE="vertical-align:top;"| | |||
{| border="0" cellpadding="5" cellspacing="0" width="850px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Fig. 2''' Total THC and CBD Measurements Differ Between Labs Across Chemotypes and Product Categories. Left column: Violin plots showing the distribution of total THC or CBD levels across labs A-F. Black lines denote median values, which are printed below the x-axis for each lab. Right column: Effect size matrices displaying the effect size of pairwise differences in distributions between labs. Matrices are color-coded according to one measure of effect size (Cohen’s d), and a second measure (Common Language) is printed for each comparison.</blockquote> | |||
|- | |||
|} | |||
|} | |||
Calculating effect sizes allows a more intuitive assessment of the magnitude of interlab differences, especially when very large sample sizes allow even trivial differences between means to reach statistical significance. For example, mean THC levels of the chemotype I flower for Lab B and Lab A were 18.4% and 17.7%, respectively (Fig. 2a and b). While this difference was highly significant due to the large sample sizes, the effect size was small (d = 0.13; see the "Methods" section). The common language effect size (CL) for this comparison was 0.54, indicating a 54% chance that a random THC measurement from Lab B will be larger than a random measurement from Lab A. In contrast, when comparing Lab F to Lab A, which reported the highest mean THC levels, the effect size was considerably larger (d = 1.28, CL = 0.82). | |||
We observed a similar pattern when comparing CBD measurements across labs for chemotype II and III flower samples (Fig. 2c and d) and THC levels for concentrates (Fig. 2e and f). The labs reporting the highest levels of THC for chemotype I flower products also reported the highest levels of CBD for other flower chemotypes and THC levels for concentrates (Fig. 2), indicating a systematic tendency for certain labs to report higher levels of cannabinoids across chemotypes and product categories. This may be explained by differences in laboratory protocols. While most labs report using [[high-performance liquid chromatography]] (HPLC) to detect cannabinoids, the details of each protocol likely differ. Alternatively, interlab differences may be driven by labs receiving distinct sets of cannabis products for testing. | |||
==References== | ==References== | ||
Revision as of 20:55, 30 March 2019
| Full article title |
The cannabinoid content of legal cannabis in Washington State varies systematically across testing facilities and popular consumer products |
|---|---|
| Journal | Scientific Reports |
| Author(s) | Jikomes, Nick; Zoorob, Michael |
| Author affiliation(s) | Leafly Holdings, Harvard University |
| Primary contact | Email: Contact author via journal |
| Year published | 2018 |
| Volume and issue | 8 |
| Page(s) | 4519 |
| DOI | 10.1038/s41598-018-22755-2 |
| ISSN | 2045-2322 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.nature.com/articles/s41598-018-22755-2 |
| Download | https://www.nature.com/articles/s41598-018-22755-2.pdf (PDF) |
|
|
This article contains rendered mathematical formulae. You may require the TeX All the Things plugin for Chrome or the Native MathML add-on and fonts for Firefox if they don't render properly for you. |
|
|
This article should not be considered complete until this message box has been removed. This is a work in progress. |
Abstract
The majority of adults in the U.S. now have state-legal access to medical or recreational cannabis products, despite their federal prohibition. Given the wide array of pharmacologically active compounds in these products, it is essential that their biochemical profile is measured and reported to consumers, which requires accurate laboratory testing. However, no universal standards for laboratory testing protocols currently exist, and there is controversy as to whether all reported results are legitimate. To investigate these concerns, we analyzed a publicly available seed-to-sale traceability dataset from Washington State containing measurements of the cannabinoid content of legal cannabis products from state-certified laboratories. Consistent with previous work, we found that commercial Cannabis strains fall into three broad chemotypes defined by the tetrahydrocannabinol:cannabidiol (THC:CBD) ratio. Moreover, we documented systematic differences in the cannabinoid content reported by different laboratories, relative stability in cannabinoid levels of commercial flower and concentrates over time, and differences between popular commercial strains. Importantly, interlab differences in cannabinoid reporting persisted even after controlling for plausible confounds. Our results underscore the need for standardized laboratory methodologies in the legal cannabis industry and provide a framework for quantitatively assessing laboratory quality.
Introduction
For millennia, Cannabis has been cultivated for medicinal, recreational, and industrial purposes.[1] Despite mounting evidence for the legitimate medical utility of cannabis products and their principal psychoactive constituents[2][3], they remain classified as Schedule I controlled substances by the U.S. federal government. Nonetheless, public opinion on legal cannabis has changed dramatically in recent years[4], and a majority of U.S. states now allow legal access to medical cannabis for approved patients, with several states also allowing recreational adult-use.[5][6] This dynamic legal landscape has given rise to a rapidly growing legal cannabis industry that offers a wide variety of products to consumers.
Because the core product of this burgeoning industry contains multiple compounds with psychoactive and medicinal properties[7], it is imperative that the major biochemical constituents of cannabis are accurately quantified, and the results made accessible to consumers. Because recreational cannabis products may differ substantially from cannabis grown for federally-sanctioned research[8] or found on the black market[9], there is a particular need to study the commercial cannabis being consumed today by millions of adults in states allowing legal adult-use consumption.
The adoption of universal industry testing standards will be crucial for comparing data across the many existing testing laboratories. However, standardized procedures have yet to be adopted, and controversy exists about whether all laboratories are accurately measuring and reporting cannabinoid content.[10] Most of these labs were not established quality control labs with a track record of testing food or pharmaceutical products, but rather started specifically to focus on cannabis products. At present, there is limited published data[8] on the content of commercial cannabis products in the U.S., including quantification of potential differences in the measurements reported across these testing laboratories. Reliable testing data will also shed light on questions important to consumers and regulators, such as whether cannabinoid levels are changing over time or differ systematically between commercial products.
To investigate these concerns, we analyzed a large dataset from Washington State’s seed-to-sale traceability system. This dataset comprises hundreds of thousands of measurements of the principal cannabinoids in commercial cannabis, including tetrahydrocannabinol (THC) and cannabidiol (CBD). These measurements are available for commercial products tested across all state-licensed laboratories since 2014, which allowed us to assess the cannabinoid composition of commercial products between laboratories, over time, and across strains.
Results
The basic chemotype landscape of commercial cannabis
Cannabis likely evolved in Central Asia, and landraces native to regions including Afghanistan, Pakistan, India and China[11] have been found to fall into three general chemotypes based on genetically-constrained THC:CBD ratios.[12][13] Consistent with previous work in landraces and commercial Dutch Cannabis[13][14], we found that commercial Cannabis grown in Washington also conforms to this pattern (Fig. 1a–c). Unlike landraces, which are more likely to fall into the chemotype III (CBD-dominant) category and generally display lower overall levels of total THC[13], most commercial Cannabis falls into the chemotype I category, characterized by relatively high total THC and low total CBD levels (Fig. 1d–f; see "Methods" section at the end for definition of total THC and CBD levels). While studying the chemotype landscape of these commercial samples, we observed striking differences in THC:CBD distributions across laboratories for both flower (Fig. 1d–f, Figure S1) and concentrates (Figure S2). This prompted us to examine interlab differences in more detail. In particular, we wished to assess whether this variation stemmed from intrinsic (e.g., methodological) differences between laboratories or from heterogeneity in the products submitted to those labs.
|
THC and CBD measurements vary widely across testing laboratories
To compare cannabinoid measurements across labs, we looked at distributions of total THC and CBD levels for the six largest laboratories by data volume for different chemotypes and product categories. These labs, referred to henceforth as labs A-F, are Confidence Analytics (Lab A), Analytical 360 (Lab B), Green Grower Labs (Lab C), Integrity Labs (Lab D), Testing Technologies (Lab E), and Peak Analytics (Lab F). We observed differences in reported values of both THC and CBD (Fig. 2). For example, the median total THC content for chemotype I flower products ranged from 17.7% to 23.2% between the labs reporting the lowest and highest THC levels, respectively (Fig. 2a; labs A-F ordered from lowest to highest median reported THC levels). Pairwise differences in mean THC content between labs were statistically significant (p < 0.001 for each pairwise comparison in Fig. 2a, two-sided t-test). To quantify the magnitude of differences between labs, we calculated the effect sizes of pairwise differences using two metrics: Cohen’s d, the standardized difference between two means[15], and a “Common Language” (CL) effect size, the probability that a random value from one sample will be greater than a random value from the other[16] (Fig. 2b; see Analytical Methods).
|
Calculating effect sizes allows a more intuitive assessment of the magnitude of interlab differences, especially when very large sample sizes allow even trivial differences between means to reach statistical significance. For example, mean THC levels of the chemotype I flower for Lab B and Lab A were 18.4% and 17.7%, respectively (Fig. 2a and b). While this difference was highly significant due to the large sample sizes, the effect size was small (d = 0.13; see the "Methods" section). The common language effect size (CL) for this comparison was 0.54, indicating a 54% chance that a random THC measurement from Lab B will be larger than a random measurement from Lab A. In contrast, when comparing Lab F to Lab A, which reported the highest mean THC levels, the effect size was considerably larger (d = 1.28, CL = 0.82).
We observed a similar pattern when comparing CBD measurements across labs for chemotype II and III flower samples (Fig. 2c and d) and THC levels for concentrates (Fig. 2e and f). The labs reporting the highest levels of THC for chemotype I flower products also reported the highest levels of CBD for other flower chemotypes and THC levels for concentrates (Fig. 2), indicating a systematic tendency for certain labs to report higher levels of cannabinoids across chemotypes and product categories. This may be explained by differences in laboratory protocols. While most labs report using high-performance liquid chromatography (HPLC) to detect cannabinoids, the details of each protocol likely differ. Alternatively, interlab differences may be driven by labs receiving distinct sets of cannabis products for testing.
References
- ↑ Grinspoon, L. (16 August 2005). "History of Cannabis as a Medicine" (PDF). MAPS. http://www.maps.org/research-archive/mmj/grinspoon_history_cannabis_medicine.pdf.
- ↑ Whiting, P.F.; Wolff, R.F.; Deshpande, S. et al. (2015). "Cannabinoids for Medical Use: A Systematic Review and meta-analysis". JAMA 313 (24): 2456–73. doi:10.1001/jama.2015.6358. PMID 26103030.
- ↑ National Academies of Sciences, Engineering, and Medicine (2017). The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. National Academies Press. doi:10.17226/24625. ISBN 9780309453073.
- ↑ Geiger, A. (12 October 2016). "Support for marijuana legalization continues to rise". Fact Tank. Pew Research Center. http://www.pewresearch.org/fact-tank/2016/10/12/support-for-marijuana-legalization-continues-to-rise. Retrieved 29 September 2017.
- ↑ Compton, W.M.; Han, B.; Highes, A. et al. (2017). "Use of Marijuana for Medical Purposes Among Adults in the United States". JAMA 317 (2): 209–11. doi:10.1001/jama.2016.18900. PMID 27992636.
- ↑ Barry, R.A.; Glantz, S. (2016). "A Public Health Framework for Legalized Retail Marijuana Based on the US Experience: Avoiding a New Tobacco Industry". PLoS Medicine 13 (9): e1002131. doi:10.1371/journal.pmed.1002131. PMC PMC5038957. PMID 27676176. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC5038957.
- ↑ Andre, C.M.; Hausman, J.-F.; Guerriero, G. (2016). "Cannabis sativa: The plant of the thousand and one molecules". Frontiers in Plant Medicine 7: 19. doi:10.3389/fpls.2016.00019. PMC PMC4740396. PMID 26870049. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC4740396.
- ↑ 8.0 8.1 Vergara, D.; Bidwell, L.C.; Gaudino, R. et al. (2017). "Compromised External Validity: Federally Produced Cannabis Does Not Reflect Legal Markets". Scientific Reports 7: 46528. doi:10.1038/srep46528. PMC PMC5395929. PMID 28422145. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC5395929.
- ↑ Morgan, C.J.; Schafer, G.; Freeman, T.P. et al. (2010). "Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: Naturalistic study". The British Journal of Psychiatry 197: 4. doi:10.1192/bjp.bp.110.077503. PMID 20884951.
- ↑ Coughlin-Bogue, T. (28 April 2017). "Leafly Investigation: Is Washington's Top Cannabis Lab Inflating THC Numbers?". Leafly. https://www.leafly.com/news/industry/leafly-investigation-washingtons-top-cannabis-lab-inflating-thc-numbers. Retrieved 13 September 2017.
- ↑ Hillig, K.W. (2005). "Genetic evidence for speciation in Cannabis (Cannabaceae)". Genetic Resources and Crop Evolution 52 (2): 161–80. doi:10.1007/s10722-003-4452-y.
- ↑ de Meijer, E.P.; Bagatta, M.; Carboni, A. et al. (2003). "The inheritance of chemical phenotype in Cannabis sativa L". Genetics 163 (1): 335–46. PMC PMC1462421. PMID 12586720. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC1462421.
- ↑ 13.0 13.1 13.2 Hillig, K.W.; Mahlberg, P.G. (2004). "A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae)". American Journal of Botany 91 (6): 966-75. doi:10.3732/ajb.91.6.966. PMID 21653452.
- ↑ Hazekamp, A.; Tejkalová, K.; Papadimitriou, S. (2016). "Cannabis: From Cultivar to Chemovar II—A Metabolomics Approach to Cannabis Classification". Cannabis and Cannabinoid Research 1 (1). doi:10.1089/can.2016.0017.
- ↑ Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). L. Eribaum Associates. ISBN 9780805802832.
- ↑ McGraw, K.O.; Wong, S.P. (1992). "A common language effect size statistic". Psychological Bulletin 111 (2): 361–65. doi:10.1037/0033-2909.111.2.361.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added.