Journal:Simultaneous quantification of 17 cannabinoids in cannabis inflorescence by liquid chromatography–mass spectrometry
| Full article title | Simultaneous quantification of 17 cannabinoids in cannabis inflorescence by liquid chromatography–mass spectrometry |
|---|---|
| Journal | Separations |
| Author(s) | Hewavitharana, Amitha K.; Gloerfelt-Tarp, Francine; Nolan, Matthew; Barkla, Bronwyn J.; Purdy, Sarah; Kretzschmar, Tobias |
| Author affiliation(s) | Southern Cross University, New South Wales Department of Primary Industries |
| Primary contact | Email: a dot hewavitharana at pharmacy dot uq dot edu dot au |
| Year published | 2022 |
| Volume and issue | 9(4) |
| Article # | 85 |
| DOI | 10.3390/separations9040085 |
| ISSN | 2297-8739 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.mdpi.com/2297-8739/9/4/85/htm |
| Download | https://www.mdpi.com/2297-8739/9/4/85/pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
With an increasing appreciation for the unique pharmacological properties associated with distinct, individual cannabinoids of Cannabis sativa, there is demand for accurate and reliable quantification for a growing number of them. Although recent methods are based on highly selective chromatography–mass spectrometry technology, most are limited to a few cannabinoids, while relying on unnecessarily sophisticated and expensive ultra-high-performance liquid chromatography and tandem mass spectrometry. Here we report an optimized, simple extraction method followed by a reliable and simple high-performance liquid chromatography (HPLC) method for separation. The detection is performed using a time-of-flight mass spectrometer that is available in most natural products research laboratories. Due to the simplicity of instrumentation, and the robustness resulting from a high resolution in the chromatography of isobaric cannabinoids, the method is well-suited for routine phytocannabinoid analysis for a range of applications. The method was validated in terms of detection and quantification limits, repeatability, and recoveries for a total of 17 cannabinoids. Detection limits were in the range 11–520 pg when using a 1 µL sample injection volume, and the recovery percentages ranged from 85% to 108%. The validated method was subsequently applied to determine cannabinoid composition in the inflorescences of several medicinal Cannabis sativa varieties.
Keywords: cannabinoids, phytocannabinoids, LC-MS, Cannabis sativa, tetrahydrocannabinol, THC, cannabidiol, CBD
Introduction
Cannabis sativa L. (Cannabis) is one of the oldest domesticated plants, cultivated for the purpose of food, fiber, psychoactives, and medicine. In recent years, its medicinal properties have gained a renewed interest given its therapeutic potential in the treatment of a variety of disease conditions. To date, over 100 different cannabinoids have been identified, with Δ9-tetrahydrocannabinol (THC), well known for its psychoactive properties, and others, such as cannabidiol (CBD), recognized for their therapeutic and medicinal value. [1,2]
Historically, cannabis samples were analyzed mostly in a legal or regulatory context for the purpose of determining Δ9-THC content. However, as more cannabinoids were identified and their pharmacological potential was examined, the quantification of cannabinoids other than Δ9-THC gained importance. Additional cannabinoids of interest now include Δ9-tetrahydrocannabinolic acid (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabigerol (CBG), cannabigerolic acid (CBGA), Δ9-tetrahydrocannabivarin (THCV), Δ9-tetrahydrocannabivarinic acid (THCVA), cannabidivarin (CBDV), and cannabidivarinic acid (CBDVA). [2,3] A recent study on virus-neutralizing capabilities of naturally occurring cannabinoids (in acid form) even found that CBDA and CBGA are capable of binding to the spike protein of SARS-CoV-2, therefore making for possible candidates for the treatment and prevention of COVID-19. [4] Clearly, accurate methods of quantification are needed to determine the concentrations of both major and minor cannabinoids of pharmacological potential in cannabis inflorescence, the source of cannabinoids in the plant.
Despite the rising demand and importance of accurate quantification of cannabinoids, the number of studies with a clear focus on analytical method development remains relatively small. This might be in part due to regulatory constraints, which necessitate specific licenses to handle this scheduled drug and limit the availability of standard compounds and test samples. Due to this, the cannabis testing industry remains poorly established, with only a relatively limited number of laboratories offering analytical services and a lack of standardized protocols for extraction and analysis. [5]
Two review articles published recently [6,7] contain critical evaluations of methods used for cannabinoid analysis and the recent trends; therefore, a detailed comparison of methods is not included here. Chromatographic techniques are generally used for the separation of cannabinoids. Liquid chromatography (LC) has gained preference over gas chromatography (GC) as LC avoids conversion of the acid forms of cannabinoids to their neutral forms, which occurs at the high temperatures used in GC. [8] Until recently, the detection of column-separated cannabinoids was predominantly carried out using UV spectrophotometry, which provides low specificity and makes baseline separation of all cannabinoids imperative. However, complete separation of structurally similar cannabinoids, especially isomeric compounds such as Δ8-THC and Δ9-THC, is challenging. [3] Due to the lack of specificity of UV detection, any unknown compound/s co-eluting at the same retention time as the target cannabinoid can cause overestimation of its concentration.
To overcome this specificity problem, the detection method of choice has been changing to mass spectrometry (MS). [6,7] As MS detection can identify molecules according to differences in mass, the chromatographic separation becomes less important. However, since some of the major cannabinoids—e.g., Δ8-THC, Δ9-THC, CBD, cannabichromene (CBC), and cannabicyclol (CBL)—have the same molecular mass, chromatographic separation is still required. Most of the methods using MS detection of cannabinoids are based on tandem mass spectrometry (MS-MS). [6,7] However, there is no real advantage of using this approach over basic MS, as cannabinoids of the same molar masses produce similar fragmentation patterns in the second MS event, thus not providing additional selectivity. Therefore, baseline separation by chromatography followed by simple MS detection is well suited for the routine analysis of cannabinoids.
According to a recent review of cannabinoid analysis, variability in extraction (solvent/s, method, time, and temperature) significantly contributes to differential analytical results. [6] Most of the recent analytical methods have used ethanol for extraction of both acidic and neutral cannabinoids [3,9], although the method used for extraction varied in terms of sample to solvent ratio, the extraction technique, and the duration of extraction. Therefore, a study of the effects of these variables on the amounts of cannabinoids extracted is warranted.
The aims of this study were to develop a simple yet effective method for the extraction of cannabinoids from cannabis inflorescences, and to develop a reliable, robust and simple liquid chromatography–mass spectrometry (LC–MS) method that can be used for routine analysis of 17 phytocannabinoids for which standards are available. In this study, a time-of-flight mass spectrometer (TOF-MS) was used as the detector. The acquisition of spectral data was by simple MS mode rather than MS-MS because there was no advantage in using tandem MS. As many natural products laboratories are equipped with a TOF instrument (primarily used for qualitative analysis), a quantification method based on a TOF-MS would facilitate adoption of this method. The extraction conditions were optimized to develop a simple and robust method to extract cannabinoids from inflorescence, without altering the composition of cannabinoids. A separation method that uses a low flow rate enabled the use of (high-performance liquid chromatography (HPLC) rather than requiring an expensive ultra-high-performance liquid chromatography (UHPLC) system. The chromatographic separation of the cannabinoids with the same molar mass was made sufficiently robust so that small changes in column and/or mobile phase do not cause the isobaric cannabinoids to co-elute.
Materials and methods
Materials
Proprietary medicinal cannabis samples of Cann Group Limited were supplied by New South Wales Department of Primary Industries (NSW DPI, Orange, Australia) under an Office of Drug Control (ODC) permit and received by Southern Cross University (SCU) under NSW Health authority. Samples of contrasting lines for THC/A and CBD/A content were used for method establishment and validation.
All cannabinoid reference standards were purchased from Novachem Pty. Ltd. (Heidelberg, Australia) as ampoules of 1,000 µg/mL solutions. The standards for (±)-Δ9-tetrahydrocannabinolic acid A (Δ9-THCA-A), tetrahydrocannabivarinic acid (THCVA), cannabidiolic acid (CBDA), cannabidivarinic acid (CBDVA), cannabigerolic acid (CBGA), cannabinolic Acid (CBNA), cannabichromenic acid (CBCA), (±)-cannabicyclol (CBL), and cannabicyclolic acid (CBLA) were supplied in acetonitrile. The standards for (−)-Δ9-tetrahydrocannabinol (Δ9-THC), (−)-Δ8-tetrahydrocannabinol (Δ8-THC), tetrahydrocannabivarin (THCV), cannabidiol (CBD), cannabidivarin (CBDV), cannabigerol (CBG), cannabinol (CBN), and cannabichromene (CBC) were supplied in methanol. Ibuprofen was purchased from Sigma-Aldrich Pty Ltd. (North Ryde, NSW, Australia). HPLC-grade ethanol (Scharlau, Scharlab S.L., Barcelona, Spain) was used for sample extractions, and LC-MS grade solvents (Honeywell Research Chemicals, Seelze, Germany) and MilliQ water (Merck Millipore, Billerica, MA, USA) were used to prepare mobile phase.
Sample preparation
Apical and mature female inflorescences were cut from the main stems, and any large stem pieces were removed. Samples were dried for approximately seven days at 24 °C under low humidity (50–58%) until a dry weight of 21% of the starting wet weight was achieved, after which they were weighed, double vacuum packed, and stored at ambient room temperature in the dark.
Two grams of inflorescence were ground to a fine powder using a 50 mL stainless steel screw top Retsch grinding canister, containing a single 20 mm stainless steel grinding ball, in a Retsch Mixer Mill MM301 (Retsch GmbH, Mettmann, Germany) at 30 rotations per second for 10 seconds. Ground samples were stored in polypropylene tubes at −20 °C until extracted for analysis (note: flash-cooling the grinding canister and ball in liquid nitrogen before adding the sample prior to grinding helped to prevent clumping of material, especially in high resin samples, thus producing a homogenous fine powder).
Optimization of the extraction
For extraction, 0.1g of ground sample was accurately weighed into a 20 mL polypropylene centrifuge tube. An aliquot of 2.50, 5.00 or 10.00 mL of 100% ethanol was accurately pipetted into the tube. Three replicates were extracted for each volume added. The mixture was sonicated in an ultrasound bath (SONICLEAN, Soniclean®, Dudley Park, Australia) at 50/60 Hz and 150 W for 15 minutes, then centrifuged at 3000 rpm (Sigma 3–16 L, Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany) for 10 minutes. The supernatant was carefully removed and stored in an Eppendorf tube at −20 °C. The extract was diluted as necessary with 100% ethanol for instrumental analysis using HPLC-MS. Volumes of injection of 1, 2 and 4 µL (for 2.50, 5.00 and 10.00 mL extractions, respectively) were used to obtain the same peak area (if each treatment were to extract the same amount). Ten cannabinoids were used for comparison (CBDV, CBDA, CBG, CBD, THCV, CBGA, THCVA, CBN, Δ9-THC, THCA). The peak areas of each cannabinoid were divided by the mass of the sample, and the peak area per gram of sample was used for comparison.
Separation and detection
The chromatographic separation of cannabinoids was performed using UHPLC on an Agilent 1290 Infinity II series system (Agilent Technologies, Santa Clara, CA, USA). The UHPLC was coupled to an Agilent 6545 high-resolution accurate mass (HRAM) QToF mass spectrometer equipped with a dual nebulizer jet stream electrospray source for ionization (Agilent) and controlled using Agilent MassHunter Acquisition Software, Tokyo, Japan (Version 10.1). Separation was achieved using an Agilent Poroshell C18 2.7 µm particle size, narrow bore, 15 cm column, held at 30 °C. The chromatographic analysis was performed using 0.1% (v/v) formic acid in 30% v/v methanol in water (mobile phase A), and 0.1% formic acid (v/v) in acetonitrile (mobile phase B), at a flow rate of 0.3 mL/min. The column was pre-equilibrated with 47% (v/v) A and 53% (v/v) B. After injection (of 1 µL sample or standard), the composition of mobile phase was maintained at 53% (v/v) B for the first 25 min. It was then changed from 53% (v/v) B to 70% (v/v) B over the next 15 minutes (to 40 minute mark), changed from 70% (v/v) B to 97% (v/v) B over the next two minutes (to 42 minute mark), and then held at 97% (v/v) B for one minute (to 43 minute mark). The column was then returned to the starting composition of 53% (v/v) B over the next one minute (to 44 minute mark). Finally, the column was re-equilibrated using 53% (v/v) B for six minutes (to 50 minute mark) prior to the next injection. The injection volume was kept low to avoid peak distortion (as explained in the discussion).
The mass spectrometer was used in MS1-positive mode to scan from m/z 190 to 370 for all samples at a scan rate of 1.5 spectra/s. The general parameters of the source were: capillary voltage 4000 V, nozzle voltage 0 V, fragmentor voltage 180 V, skimmer voltage 45 V and octopole RF Peak voltage 750 V, nebulizer pressure 20 psi, drying gas 10 L/minute, drying gas temperature 325 °C, sheath gas flow 12, and sheath gas temperature 400 °C.
Data analysis and calculations
Data analysis was performed using Agilent MassHunter Quantitative Analysis (for QToF) Software, Tokyo, Japan (Version 10.1). Each cannabinoid in a chromatogram of standard compounds was identified based on its spectrum and the retention time. A processing method was prepared to extract each peak based on the m/z value of each compound within a ±0.03 m/z window. Peaks were integrated and exported to EXCEL for calculations. External standard calibration was performed using a five-point calibration curve up to 12.5 µg/mL concentration with 1 µL injections. The regression values obtained (slope and intercept) in EXCEL were formatted to contain the same number of significant figures as the peak area values to avoid rounding errors. Standards used in calibration were used as unknowns/samples to predict concentrations and estimate the validity of the calibration. Concentrations calculated for the samples injected were checked to ensure that they were within the calibration/linear range. If not, they were re-run after dilution. After multiplying by the dilution factor, concentrations were expressed as µg/g of the sample using the exact mass of the sample used in extraction. Dilution factors (of the 5 mL extract) used for minor and major cannabinoids in this study were 2.5 and 200, respectively. A pooled sample of ground inflorescence was prepared using a mixture of high-THC and high-CBD plant material and used as the quality control (QC) sample with each batch of the samples analyzed.
Extraction method used for routine analysis
A sample of 0.1g ground cannabis inflorescence was accurately weighed into a 20 mL polypropylene centrifuge tube. A 5.00 mL aliquot of 100% ethanol was accurately pipetted into the tube. The mixture was sonicated in an ultrasound bath at 50/60 Hz and 150 W for 30 minutes, then centrifuged at 3,000 rpm (Sigma 3–16 L, Germany) for 10 minutes. The supernatant was carefully removed and stored in a 2 mL Eppendorf tube at −20 °C. The extract was diluted as necessary (below) with 100% ethanol prior to the instrumental analysis using HPLC-MS.
Preparation of samples for major cannabinoids: THC, THCA, CBD, CBDA
The extract was diluted 80 times by mixing 20 µL sample extract and 1580 µL ethanol. The extract was then further diluted by mixing 400 µL of the diluted extract, 40 µL of 0.025 mg/mL Ibuprofen, and 560 µL ethanol in an HPLC vial to be used for LC-MS analysis. This resulted in a 200 times diluted extract containing 1 µg/mL Ibuprofen (internal standard).
Preparation of samples for minor cannabinoids
The extract was diluted by mixing 400 µL of the extract, 40 µL of 0.025 mg/mL Ibuprofen, and 560 µL ethanol in an HPLC vial to be used for LC-MS analysis. This resulted in a 2.5 times diluted extract containing 1 µg/mL Ibuprofen (internal standard).
Preparation of standard solutions
A 0.05 mg/mL solution of 17 cannabinoid standards was prepared by mixing 100 µL each of the 17 cannabinoid stock standards (1 mg/mL), and 300 µL of HPLC grade ethanol in a 2 mL Eppendorf tube. A 0.025 mg/mL mix of standards was prepared by diluting the 0.05 mg/mL solution and used as the working standard stock solution.
Ibuprofen was weighed into a 1.5 mL Eppendorf tube and dissolved in an appropriate volume of HPLC grade ethanol to make a 1 mg/mL stock solution. This solution was diluted with an appropriate volume of ethanol to make the 0.025 mg/mL working stock standard.
Five calibration standards were prepared by diluting the appropriate volumes of 0.025 mg/mL mix of 17 standards, and 0.025 mg/mL Ibuprofen to contain 2.5, 5, 7.5, 10, and 12.5 µg/mL cannabinoids and 1 µg/mL Ibuprofen (internal standard).
Method validation
Linearity for each cannabinoid was estimated by injecting 1µL each of five mixed standard solutions containing 0.5 to 12.5 µg/mL of the 17 cannabinoids. The linearity was assessed by plotting the peak area of each cannabinoid versus the concentration. Limit of detection (LOD) and limit of quantification (LOQ) for each cannabinoid was determined based on the peak heights (and then the concentrations) corresponding to 3× noise and 10× noise, respectively. Repeatability was estimated by analyzing seven individual 0.1 g ground cannabis inflorescence samples (that were prepared by pooling samples containing high CBD and high THC levels), followed by the calculation of relative standard deviation of the concentrations determined for each cannabinoid. Recovery was determined by using the concentrations of each cannabinoid in spiked and un-spiked extracts using seven of the individual extracts prepared for the repeatability experiment. Each extract was spiked to give an added concentration that was below the mid-point of the calibration (linear) range.
Results and discussion
Sample preparation
A significantly larger (than the amount used for analysis) amount of inflorescence (2 g) was ground in order to minimize effects of within sample variation due to the architecture/morphology of the inflorescence (containing bracts, stigma, sugar leaves, stem remnants, etc.) of varying trichome density. After homogenization by grinding, a representative 0.1 g sample was extracted for analysis.
Optimization of the extraction
Ethanol has been the most common solvent of choice in recent studies of cannabinoid analysis, and the high extracting efficiency of ethanol is attributed to the high affinity of ethanol to the cannabinoid molecular structure. [6] Important advantages of using ethanol extraction for cannabinoid analysis are its general availability, low toxicity, low cost, and easy storage and handling. Furthermore, the concentrations determined by the analytical methods that used ethanol for extraction are likely in the range of concentrations used for actual extraction in formulations intended for human consumption, as ethanol is a common extraction solvent in preparations. An extraction that does not involve heat is more appropriate for cannabinoid extraction because heat can change the ratio of acid forms to neutral forms [8], thereby altering the composition of cannabinoids in the sample prior to analysis.
An optimization study of the extraction of cannabinoids from dried, ground cannabis inflorescence, using factorial design, found that the extraction solvent (80% methanol vs methanol-chloroform mixture), technique of extraction, and the time of extraction beyond 15 minutes did not significantly affect the amounts of cannabinoids extracted. The most significant variable that affected the amounts extracted was the sample to solvent ratio. [10] In recent methods that used ethanol as the extraction solvent, the ratio (grams of dried inflorescence/mL ethanol) varied from 1/100 [9,11] to 1/15. [3] Therefore, a study of the effect of this ratio on the amounts of cannabinoids extracted was warranted in order to establish an optimum extraction procedure prior to instrumental analysis. A systematic study of the effects of solvents other than the ones studied in the previous study [10], and extraction times below 15 minutes, was beyond the scope of this study.
In this study, the effect of the ratio "grams of ground inflorescence / mL ethanol" on the amount of cannabinoids extracted was studied using 0.1g of dried inflorescence in 2.5, 5, and 10 mL volumes of ethanol (ratios of 1/25, 1/50, and 1/100, respectively), using triplicate extractions for each ratio. Volumes of injection of 1, 2, and 4 µL (respectively) were used to obtain the same peak area (if each treatment were to extract the same amount). Ten cannabinoids that were present in significant amounts in the sample were used for comparison (CBDV, CBDA, CBG, CBD, THCV, CBGA, THCVA, CBN, Δ9-THC, THCA). Peak area per gram of sample was used for comparison.
According to the results of one-way ANOVA (plus a Duncan’s multiple range test for the means), sample/solvent ratio of 1/100 extracted significantly lower (p < 0.05) amounts of all cannabinoids in comparison to the other two ratios. The amounts extracted with the ratios 1/25 and 1/50 were not significantly different (at p = 0.05) for all cannabinoids. However, the precision (based on the coefficient of variance) was better with the ratio 1/50. Therefore, that ratio (0.1 g of ground inflorescence in 5 mL ethanol) was used for all extractions in this study.
Optimization of chromatographic separation and mass spectrometry
Chromatographic separation for all cannabinoids was optimized, with the main emphasis on the baseline separation of the critical isomeric pair of Δ8-THC and Δ9-THC. Chromatographic separation with good resolution was also targeted for individual cannabinoids in two groups of isobaric compounds: CBD, Δ8-THC, Δ9-THC, CBC, CBL (m/z = 315.2) and CBDA, THCA, CBLA, CBCA (m/z = 359.2). In order to conduct these separations, a column with small particle size and/or maximum possible length was important. However, because of the aim of making this separation suitable for HPLC rather than UHPLC, it was decided to use a poroshell-type column (a thin layer of porous stationary phase is coated on solid silica cores) that can produce resolutions similar to sub-2 µm body-porous stationary phase particles used in UHPLC columns, without having to use very high pressure. As the longest available LC-MS column (15 cm) was used in this separation, the high pressure required was compensated by using a low flow rate (0.3 mL/min). Although the run time could have been shortened by using a higher flow rate, the flow rate was kept low in order to operate the LC in HPLC mode rather than UHPLC mode, particularly because our aim was to produce a method that can be run on a simple HPLC system.
The polarity of a C18 column is suitable for the separation when a low pH is used (when the acid forms are not ionized to produce highly polar carboxylates). The detector (MS) sensitivities for neutral compounds were much higher than those for acid forms at low pH. Another advantage of using low pH was, that the neutral forms of cannabinoids naturally occur at much lower concentrations compared to the acid forms. Therefore, the difference in sensitivities was well suited for analyzing a mixture of acids and neutrals without having to make additional dilutions to quantify the range of cannabinoids of interest. Formic acid (0.1%) was used to achieve a low pH of around three in the mobile phase.
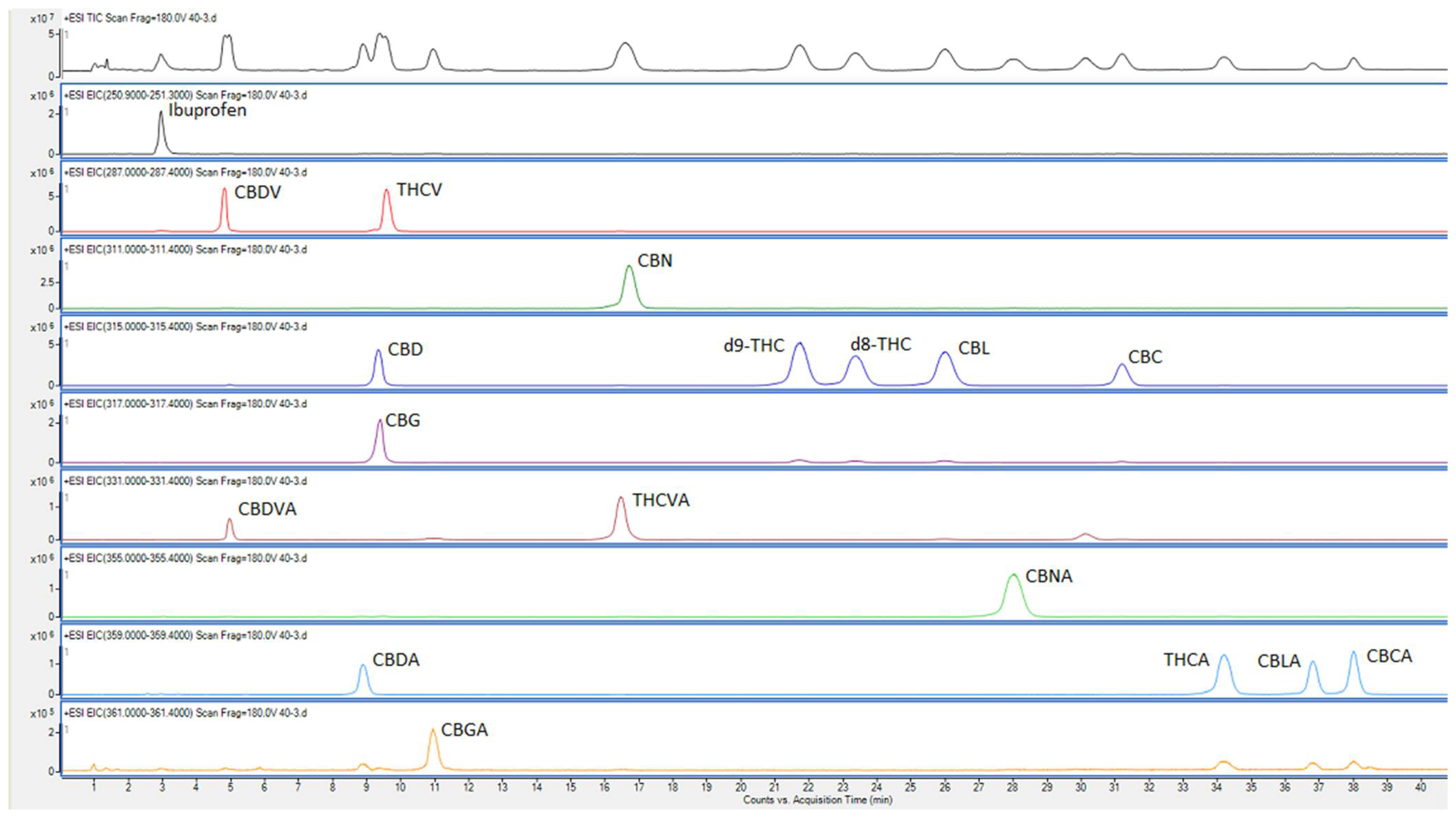
Organic modifiers—methanol and acetonitrile, from two different classes of selectivity—were tested in different ratios and combinations to improve the selectivity factor of the separations. [12] The optimal separation of all 17 cannabinoids was achieved within the isocratic section at the beginning of the gradient using 53% v/v methanol/water (with 0.1% formic acid) as mobile phase A, and acetonitrile (with 0.1% formic acid) on a Poroshell C18 column with a particle size of 2.7 µm (as detailed under Section 2 above) (Figure 1). The m/z values used for detection and the retention times for each cannabinoid are listed in Table 1. Since the samples and standards were in 100% organic solvent (ethanol), and the start of the gradient was only about 53% organic (weaker eluent strength), the chromatographic peaks were prone to distortion. With the column size and flow rate used in this study, we found that peak distortion could be prevented as long as the volume of injection was kept below 4 µL. The injection volume was kept at 1 µL throughout this study.
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added.