Difference between revisions of "Journal:Quality control of cannabis inflorescence and oil products: Response factors for the cost-efficient determination of ten cannabinoids by HPLC"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 43: | Line 43: | ||
An experimental basis for the entourage effect is provided by [[wikipedia:Murinae|murine]] studies, which have demonstrated that binary combinations with acidic cannabinoids increase bioavailability, [[potency]], and efficacy of neutral cannabinoids in epilepsy models. [16—18] Even authors exclusively preoccupied with neutral cannabinoids have demonstrated synergistic binary combinations. [19—21] Clinical evidence is also mounting, with a recent meta-analysis on observational studies of epileptic patients concluding that crude [[Cannabis concentrate|cannabis extracts]] yielded a greater reduction in seizure frequency and had fewer side-effects than equivalent doses of purified CBD. [22] However, as most extracts were only characterized to the extent of standardizing the CBD dose, information about other cannabinoids was absent or based on inference. Consequently, the authors’ attribution of the differences between the extracts and purified CBD to the entourage effect was speculative. It was not possible to evaluate if the effects of the other cannabinoids added together, comparable to merely increasing the dose of CBD, or if they magnified the effect to surpass what CBD could achieve alone. Evidently, to progress beyond studies of binary combinations or poorly characterized extracts, routine analyses capable of quantifying panels of cannabinoids could help to better inform the design and interpretation of future studies that investigate the entourage effect. A clinical understanding of this effect might subsequently inform the extent to which cannabinoids are screened during cannabis product quality control. | An experimental basis for the entourage effect is provided by [[wikipedia:Murinae|murine]] studies, which have demonstrated that binary combinations with acidic cannabinoids increase bioavailability, [[potency]], and efficacy of neutral cannabinoids in epilepsy models. [16—18] Even authors exclusively preoccupied with neutral cannabinoids have demonstrated synergistic binary combinations. [19—21] Clinical evidence is also mounting, with a recent meta-analysis on observational studies of epileptic patients concluding that crude [[Cannabis concentrate|cannabis extracts]] yielded a greater reduction in seizure frequency and had fewer side-effects than equivalent doses of purified CBD. [22] However, as most extracts were only characterized to the extent of standardizing the CBD dose, information about other cannabinoids was absent or based on inference. Consequently, the authors’ attribution of the differences between the extracts and purified CBD to the entourage effect was speculative. It was not possible to evaluate if the effects of the other cannabinoids added together, comparable to merely increasing the dose of CBD, or if they magnified the effect to surpass what CBD could achieve alone. Evidently, to progress beyond studies of binary combinations or poorly characterized extracts, routine analyses capable of quantifying panels of cannabinoids could help to better inform the design and interpretation of future studies that investigate the entourage effect. A clinical understanding of this effect might subsequently inform the extent to which cannabinoids are screened during cannabis product quality control. | ||
Several published methods are available for the separation and quantification of cannabinoids, with a variety of limitations which constrain their routine use. For the analysis of neutral cannabinoids, [[gas chromatography]] (GC) is simple, sensitive, and provides acceptable resolution. [23] However, GC is not immediately suitable for acidic cannabinoids, as they are poorly | Several published methods are available for the separation and quantification of cannabinoids, with a variety of limitations which constrain their routine use. For the analysis of neutral cannabinoids, [[gas chromatography]] (GC) is simple, sensitive, and provides acceptable resolution. [23] However, GC is not immediately suitable for acidic cannabinoids, as they are poorly volatilized and rapidly undergo thermal decarboxylation into neutral cannabinoids. [24] Fortunately, this limitation can be surmounted by trimethylsilyl derivatization of the labile acid group. [24,25] Alternatively, some analysts have adopted [[High-performance liquid chromatography|liquid chromatography]] (LC) for the separation of cannabinoids in medicinal cannabis. Following separation by LC, detection can be achieved by [[mass spectrometry]] (MS) or by [[Photodiode#Photodiode array|photodiode array]] (PDA). The MS detector enables the peak identity confirmation from their fragmentation patterns and relative ratios [26], and it is sufficiently specific to recognize coeluting impurities in complex matrices. [24] However, the required technical expertise, operation, and maintenance costs prohibit the use of MS for the routine analysis of cannabinoids. The [[ultraviolet–visible spectroscopy]] (UV-Vis) PDA detectors are much cheaper, require less operator expertise, and are widely available. Since cannabinoids contain UV [[chromophore]]s [27], they are amenable to PDA detection. Moreover, the UV spectra may assist with compound identity confirmation and the measurement of peak purity, which aids in quantification. | ||
Whichever detector is used, the elevated cost and limited availability of certified analytical reference standards for some cannabinoids remain impediments to their analysis. The cost can exceed $200 AUD per mg, and newly identified pharmacological leads in cannabis are possibly more expensive with significantly longer shipping times. To surmount this, some analysts have performed [[wikipedia:Stereoselectivity|stereoselective]] microscale syntheses to obtain cannabinoids in a timelier manner [28,29], but this is beyond the remit of a typical QC lab. If the analysis of such cannabinoids is to become routine, the cost for their quantification must be mitigated. | Whichever detector is used, the elevated cost and limited availability of certified analytical reference standards for some cannabinoids remain impediments to their analysis. The cost can exceed $200 AUD per mg, and newly identified pharmacological leads in cannabis are possibly more expensive with significantly longer shipping times. To surmount this, some analysts have performed [[wikipedia:Stereoselectivity|stereoselective]] microscale syntheses to obtain cannabinoids in a timelier manner [28,29], but this is beyond the remit of a typical QC lab. If the analysis of such cannabinoids is to become routine, the cost for their quantification must be mitigated. | ||
| Line 61: | Line 61: | ||
====Instrumentation and analytical method==== | ====Instrumentation and analytical method==== | ||
A Shimadzu Prominence-i LC-2030C 3D Plus HPLC-PDA (Rydalmere, NSW, AU)—comprising of a low-pressure quaternary solvent system, an auto sampler, and a PDA detector—was used. Shimadzu LabSolutions (v5.93A) was used for instrument control, data acquisition, and processing. A Phenomenex Luna C18(2) (150 × 4.6 mm × 5 µm) analytical column with a Security C18 (20 × 4.6 mm × 5 µm) guard column (Lane Cove West, NSW, AU) was employed to achieve reversed phase separation. The column was maintained at 40 °C, with a mobile phase flow rate of 2.5 mL/min. The injection volume was 10 µL, and all standards and samples were injected in duplicate. Gradient elution employing mobile phase A (Milli-Q water buffered with 20 mM ammonium formate and 0.1% formic acid), mobile phase B (acetonitrile) and mobile phase C (methanol buffered with 10 mM ammonium formate and 0.05% formic acid) was used. The gradient program is | A Shimadzu Prominence-i LC-2030C 3D Plus HPLC-PDA (Rydalmere, NSW, AU)—comprising of a low-pressure quaternary solvent system, an auto sampler, and a PDA detector—was used. Shimadzu LabSolutions (v5.93A) was used for instrument control, data acquisition, and processing. A Phenomenex Luna C18(2) (150 × 4.6 mm × 5 µm) analytical column with a Security C18 (20 × 4.6 mm × 5 µm) guard column (Lane Cove West, NSW, AU) was employed to achieve reversed phase separation. The column was maintained at 40 °C, with a mobile phase flow rate of 2.5 mL/min. The injection volume was 10 µL, and all standards and samples were injected in duplicate. Gradient elution employing mobile phase A (Milli-Q water buffered with 20 mM ammonium formate and 0.1% formic acid), mobile phase B (acetonitrile) and mobile phase C (methanol buffered with 10 mM ammonium formate and 0.05% formic acid) was used. The gradient program is summarized in Table 1, which includes two minutes of column rinse with the organic phases and two minutes of re-equilibration at the starting condition. During each run, the PDA was set to acquire data from 190 to 800 nm and the [[Chromatography|chromatograms]] was visualized at 232 nm. | ||
{| | {| | ||
| Line 130: | Line 130: | ||
==Results and discussion== | ==Results and discussion== | ||
===Method development=== | ===Method development=== | ||
To optimize sample preparation, a variety of extraction solvents were tested with duplicate extractions. The solvents trailed were methanol, ethanol, acetonitrile, ethyl acetate, methanol:water (1:1 v/v), and acetonitrile:methanol (4:1 v/v). Cannabinoid peak areas were maximized by ethyl acetate and acetonitrile:methanol. However, due to markedly mismatching the initial mobile phase condition, ethyl acetate gave rise to significant band broadening. Thus, acetonitrile:methanol (4:1 v/v) was selected as the extraction solvent, which is consistent with other extraction optimization reports. [25,31] | |||
The CV contribution of the method preparation procedure to the total uncertainty was determined by performing six replicate extractions and analysis of a single cannabis inflorescence sample. Grinding the inflorescence to pass through a < 710 µm sieve before subsampling achieved a CV range of 1.2 to 3.6%. When subsampling without grinding, the CV unacceptably ranged from 7.6 to 23.6%, thus indicating the importance of preparing a homogeneous sample. | |||
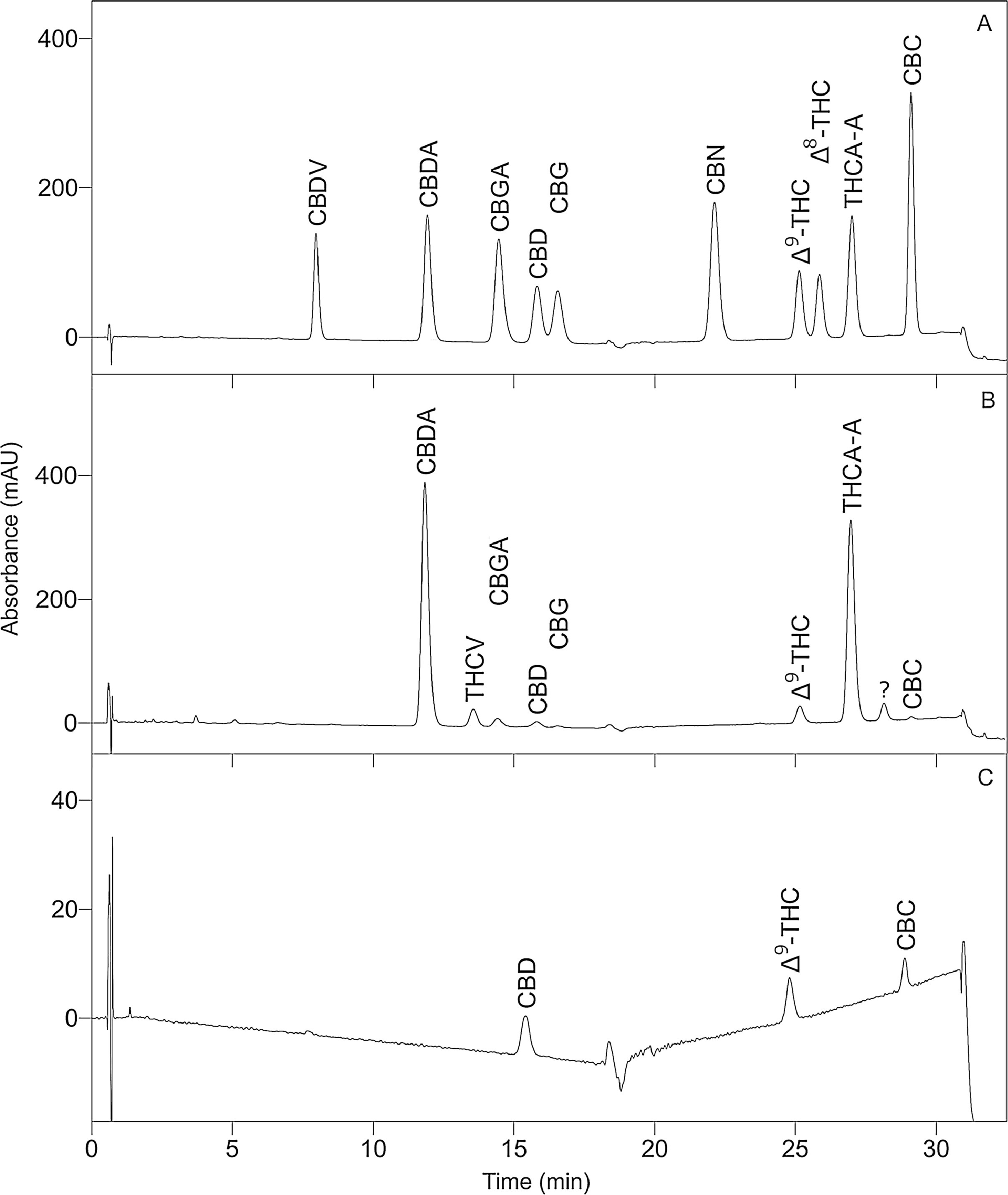
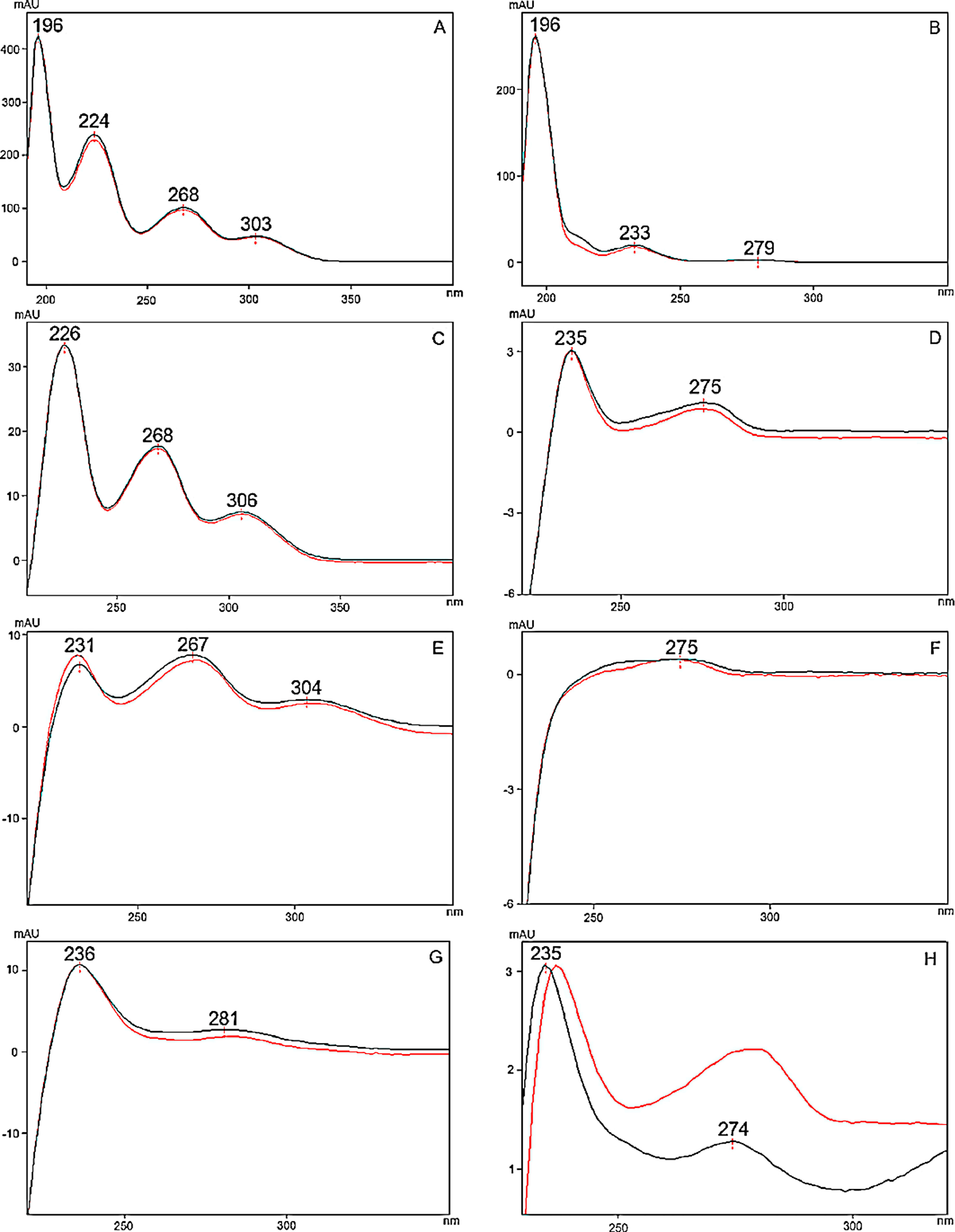
To optimize the chromatographic conditions, the method was iteratively developed. Baseline separation was achieved for eight of the 10 cannabinoid standards (resolution > 2.0); however, the CBD and CBG standard peaks overlapped slightly (resolution > 1.5, in all batches), as shown in Fig. 1A. Likewise, acceptable separation of cannabinoids in the extracts of cannabis inflorescence and cannabis oil were demonstrated in Fig. 1B and C, respectively. Whilst most matrix components eluted before the cannabinoids, a compound in inflorescence samples was observed to elute between CBDA and CBGA. This peak was identified to be tetrahydrocannabivarin (THCV, t<sub>R</sub> 13.70 minutes) by comparison with the UV spectrum and retention time obtained for the THCV standard. THCV was not included in the present method validation study as it was not part of the original selected set of analytes. When the analytes were sufficiently abundant in the sample, the UV spectra of their peaks were compared to that of the standard. As shown in Fig. 2, spectra superimposed closely, indicating good peak purity. | |||
[[File:Fig1 Hall TalantaOpen2022 5.jpg|600px]] | |||
{{clear}} | |||
{| | |||
| STYLE="vertical-align:top;"| | |||
{| border="0" cellpadding="5" cellspacing="0" width="600px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Fig. 1.''' Representative chromatograms at 232 nm. '''A''' Mixed cannabinoid standard. '''B''' Cannabis inflorescence. '''C''' Cannabis oil.</blockquote> | |||
|- | |||
|} | |||
|} | |||
[[File:Fig2 Hall TalantaOpen2022 5.jpg|700px]] | |||
{{clear}} | |||
{| | |||
| STYLE="vertical-align:top;"| | |||
{| border="0" cellpadding="5" cellspacing="0" width="700px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Fig. 2.''' Spectra of cannabinoid peaks recorded from the standards (black) and inflorescence sample (red). '''A''' THCA-A. '''B''' Δ<sup>9</sup>-THC. '''C''' CBDA.''' D''' CBD. '''E''' CBGA. '''F''' CBG. '''G''' CBC. '''H''' Δ<sup>9</sup>-THCV. Note that different abundances relative to an uneven baseline absorbance accounts for the imperfect overlap.</blockquote> | |||
|- | |||
|} | |||
|} | |||
To optimise PDA detection, wavelengths corresponding to the λ<sub>max</sub> of the different cannabinoids, specifically 210, 232, and 270 nm, were considered. Whilst 210 nm has been used in other studies [24,25,32], it produced a sloping baseline in the present study due to the use of methanol (UV cut-off 210 nm) rather than exclusively using acetonitrile (UV cut-off 190 nm) as the organic component of the mobile phase. Instead, it was found that visualizing the chromatogram at 232 nm gave the best compromise between sensitivity and baseline noise. Some studies used 270 nm to improve sensitivity for the acidic cannabinoids [33], but this higher sensitivity is not required due to their relatively high abundance in the inflorescence samples. This high abundance was anticipated as acidic cannabinoids are the secondary metabolites synthesized in cannabis, whereas the neutral forms are produced by spontaneous decarboxylation. [34] | |||
===Linearity and calibration range=== | |||
Considering the seven mixed-standard dilutions prepared over the 1.6 to 250 µg/mL range, replicates at the lowest concentration for most cannabinoids deviated from their mean by >5%, indicating significant baseline noise at this level. Accordingly, calibration curves were constructed using the six standards from 3.1 to 250 µg/mL. The corresponding linear equations and their R<sup>2</sup> are summarized in Table 2. Good linearity was obtained, with R<sup>2</sup> > 0.9999 for all the analytes. The magnitudes of the intercepts were compared to the integrations at the working standard concentration, and all were deemed insignificant, as 3% of the working standard integration was greater than the absolute value of the intercept. Thus, the calibration equations were linear and passed sufficiently close to the origin for RRF values determined from the working standard concentration to be reliable. | |||
Revision as of 22:38, 12 December 2022
| Full article title | Quality control of cannabis inflorescence and oil products: Response factors for the cost-efficient determination of ten cannabinoids by HPLCn |
|---|---|
| Journal | Talanta Open |
| Author(s) | Hall, Damian R.; Sinclair, Justin S.; Bhuyan, Deep J.; Khoo, Cheang; Li, Chun G.; Sarris, Jerome; Low, Mitchell |
| Author affiliation(s) | NICM Health Research Institute, Wentworth Institute, Florey Institute of Neuroscience and Mental Health |
| Primary contact | Email: Mitchell dot Low at westernsydney dot edu dot au |
| Year published | 2022 |
| Volume and issue | 5 |
| Article # | 100112 |
| DOI | 10.1016/j.talo.2022.100112 |
| ISSN | 2666-8319 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.sciencedirect.com/science/article/pii/S2666831922000315 |
| Download | https://www.sciencedirect.com/science/article/pii/S2666831922000315/pdfft (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
The quality control (QC) of medicinal cannabis should include quantification of as many cannabinoids as practicable in a routine analytical laboratory, to accurately reflect the quality of the product. However, the cost and availability of some cannabinoid standards is an impediment to their routine use. This work seeks to overcome this obstacle by analyzing samples using relative retention times (RRT) and relative response factors (RRF), relative to cannabidiol (CBD) and cannabidiolic acid (CBDA) reference standards which are readily available. A high-performance liquid chromatography-photodiode array method was developed to quantify 10 cannabinoids—tetrahydrocannabinol (Δ9-THC), delta-8-Tetrahydrocannabinol (Δ8-THC), delta-9-Tetrahydrocannabinolic acid A (THCA-A), cannabinol (CBN), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabichromene (CBC), cannabidivarin (CBDV), cannabigerol (CBG), and cannabigerolic acid (CBGA)—in dried cannabis inflorescence and cannabis oil. This method was validated according to International Conference on Harmonization (ICH) guidelines.
The proposed method has detection limits ranging from 20 to 78 µg/g, which provided sufficient sensitivity for the panel of cannabinoids. Non-cannabinoid surrogate matrices were used for spike recovery studies to determine method accuracy; analyte recoveries for the inflorescence and oil ranged from 90.1 to 109.3% (inflorescence mean, 100.9%; oil mean, 99.6%). The RRT and RRF values, determined independently by three analysts, were comparable, indicating the method is robust. The validity of analysis using RRT and RRF was further confirmed by testing six inflorescence samples, as it was found that concentrations above the order of magnitude of the limit of quantitation (LoQ) agreed satisfactorily (range, 95.0 to 111.9%; mean, 100.0%) with the concentrations obtained through the conventional approach of multipoint calibration using pure standards. The proposed method is therefore suitable for the rapid and simple determination of a panel of 10 cannabinoids without having to repeatedly purchase every expensive pure standard. Accordingly, analysts in the medicinal cannabis field may explore the use of RRF and RRT for their methods and instruments.
Keywords: liquid chromatography, cannabis, cannabinoid, response factor, relative retention time
Introduction
Despite an extensive history of use as a medicinal plant spanning ancient cultures [1–3], cannabis use is contentious in many jurisdictions, as it has been considered a social drug of abuse since the mid-1930s. [4,5] Over the last two decades, meaningful legal, sociocultural, and economic change has led to the establishment of medicinal cannabis research programs in several countries, which have validated the therapeutic use of cannabis for indications, including chronic neuropathic pain, certain intractable epilepsies, the vomiting and spasticity of multiple sclerosis, and chemotherapy-induced nausea. [6] Further to this, the use of medicinal cannabis has expanded into pediatric and vulnerable patient groups [7–9], and regulated markets for recreational use have developed in some jurisdictions. Accordingly, quality control (QC) across the supply chain is increasingly important to ensure that cannabis products are safe and have well-defined chemical and therapeutic profiles.
Critically, the complex relationship between chemical profiles and therapeutic activity requires further exploration. Presently, the activities of the three most abundant neutral cannabinoids—tetrahydrocannabinol (Δ9-THC), cannabidiol (CBD), and cannabigerol (CBG)—have been studied closely, exhibiting numerous properties, including analgesic, anticonvulsant, and anti-inflammatory characteristics. [6,10] However, the full potential of medicinal cannabis may not be realized without leveraging the full diversity of cannabinoids. Over 140 cannabinoids have been identified, many of which have their own inherent pharmacological properties. [11,12] This includes the acidic cannabinoids, which have significant anticonvulsant activities, contrary to the historical perspective that they were inert precursors which only acquired activity after decarboxylating into the neutral cannabinoids. [13] Furthermore, the complexity of cannabis increases geometrically under the "entourage effect," which postulates that cannabinoids interact to modulate their therapeutic effects. [14,15]
An experimental basis for the entourage effect is provided by murine studies, which have demonstrated that binary combinations with acidic cannabinoids increase bioavailability, potency, and efficacy of neutral cannabinoids in epilepsy models. [16—18] Even authors exclusively preoccupied with neutral cannabinoids have demonstrated synergistic binary combinations. [19—21] Clinical evidence is also mounting, with a recent meta-analysis on observational studies of epileptic patients concluding that crude cannabis extracts yielded a greater reduction in seizure frequency and had fewer side-effects than equivalent doses of purified CBD. [22] However, as most extracts were only characterized to the extent of standardizing the CBD dose, information about other cannabinoids was absent or based on inference. Consequently, the authors’ attribution of the differences between the extracts and purified CBD to the entourage effect was speculative. It was not possible to evaluate if the effects of the other cannabinoids added together, comparable to merely increasing the dose of CBD, or if they magnified the effect to surpass what CBD could achieve alone. Evidently, to progress beyond studies of binary combinations or poorly characterized extracts, routine analyses capable of quantifying panels of cannabinoids could help to better inform the design and interpretation of future studies that investigate the entourage effect. A clinical understanding of this effect might subsequently inform the extent to which cannabinoids are screened during cannabis product quality control.
Several published methods are available for the separation and quantification of cannabinoids, with a variety of limitations which constrain their routine use. For the analysis of neutral cannabinoids, gas chromatography (GC) is simple, sensitive, and provides acceptable resolution. [23] However, GC is not immediately suitable for acidic cannabinoids, as they are poorly volatilized and rapidly undergo thermal decarboxylation into neutral cannabinoids. [24] Fortunately, this limitation can be surmounted by trimethylsilyl derivatization of the labile acid group. [24,25] Alternatively, some analysts have adopted liquid chromatography (LC) for the separation of cannabinoids in medicinal cannabis. Following separation by LC, detection can be achieved by mass spectrometry (MS) or by photodiode array (PDA). The MS detector enables the peak identity confirmation from their fragmentation patterns and relative ratios [26], and it is sufficiently specific to recognize coeluting impurities in complex matrices. [24] However, the required technical expertise, operation, and maintenance costs prohibit the use of MS for the routine analysis of cannabinoids. The ultraviolet–visible spectroscopy (UV-Vis) PDA detectors are much cheaper, require less operator expertise, and are widely available. Since cannabinoids contain UV chromophores [27], they are amenable to PDA detection. Moreover, the UV spectra may assist with compound identity confirmation and the measurement of peak purity, which aids in quantification.
Whichever detector is used, the elevated cost and limited availability of certified analytical reference standards for some cannabinoids remain impediments to their analysis. The cost can exceed $200 AUD per mg, and newly identified pharmacological leads in cannabis are possibly more expensive with significantly longer shipping times. To surmount this, some analysts have performed stereoselective microscale syntheses to obtain cannabinoids in a timelier manner [28,29], but this is beyond the remit of a typical QC lab. If the analysis of such cannabinoids is to become routine, the cost for their quantification must be mitigated.
To this end, this study aimed to develop and validate a high-performance liquid chromatography-photodiode array (HPLC-PDA) method for the determination of 10 cannabinoids in medicinal cannabis inflorescence and oil and to explore the feasibility of using relative retention times (RRT) for peak identification and relative response factors (RRF) for their quantification. By this approach, an initial once-off purchase of all the standards was required to establish the RRT and RRF between the cannabinoids and the reference compounds. This included CBD as a reference for neutral cannabinoids, and cannabidiolic acid (CBDA) as a reference for acidic cannabinoids; they were chosen as they are cheaper and available in many jurisdictions. Subsequently, the method may be routinely used in QC laboratories for the quantification of a panel of 10 cannabinoids, requiring only sparing amounts of the reference compounds.
Materials and methods
Reagents and standards
LC-grade acetonitrile and methanol (purity >99.9%) were sourced from Honeywell (North Ryde, NSW, AU). Formic acid (>98%), ammonium formate (>98%), and dichloromethane (>99.8%) were sourced from Sigma-Aldrich (Castle Hill, NSW, AU). Ultra-pure water (resistivity >18.0 MΩ.cm) was obtained from a Milli-Q Direct 9 system (Sigma-Aldrich). A mixed 250 µg/mL standard of neutral cannabinoids—Δ9-THC, CBD, CBG, delta-8-Tetrahydrocannabinol (Δ8-THC), cannabinol (CBN), cannabichromene (CBC), and cannabidivarin (CBDV)—and acidic cannabinoids—CBDA, delta-9-Tetrahydrocannabinolic acid A (THCA-A), and cannabigerolic acid (CBGA)—in acetonitrile was produced by Cayman Chemical Company (Ann Arbor, MI, USA) and distributed by Sapphire Bioscience (Redfern, NSW, AU). Primary standards of single cannabinoids were also from the Cayman Chemical Company: CBN, THCA-A, and CBDA were obtained as 1000 µg/mL acetonitrile solutions; CBDV, CBC and tetrahydrocannabivarin (THCV) were obtained as 1000 µg/mL methanol solutions; and CBD, Δ9-THC, Δ8-THC, CBG, and CBGA were sourced as anhydrous solids. All standards were stored at −20 °C and allowed to come to room temperature in a desiccator before use.
An extraction solvent of acetonitrile:methanol (4:1 v/v) was prepared fresh daily, as required for the dilution of standards and the extraction of samples. For the conventional multipoint calibration curve, a 50 µg/mL dilution was prepared directly from the original 250 µg/mL mixed standard, and serial dilutions with a factor of two covered the concentration range of 1.6 to 25 µg/mL. For the quantification by RRF, a 25 µg/mL working standard of CBD and CBDA was prepared from their primary standards. This working standard concentration was chosen to be in the same order of magnitude as the sample concentration obtained when a 10 mg/g CBD oil is extracted according to the "sample preparation" subsection, below. All dilutions were prepared in the extraction solvent.
Sample preparation
Six air-dried and coarsely ground cannabis inflorescences, denoted as samples A-F, were provided by Little Green Pharma (Kings Park, WA, Australia). To ensure representative sampling of the biomass, the inflorescences were mechanically processed to pass through a 24-mesh sieve (710 µm openings). Residual water content (mean 5.5% w/w; CV <3%) was verified by drying triplicate sub-samples (∼ 800 mg) of the air-dried inflorescence over phosphorous pentoxide in a vacuum desiccator; drying was to a constant mass (<2 mg difference between days 7 and 8). For the analysis of cannabinoids, air-dried samples (400 mg) prepared in extraction solvent (25 mL) were sonicated in a Powersonic 420 ultrasonic bath (Thermoline Scientific; Sydney, NSW, Australia) on low power at room temperature for 30 minutes. Extracts were passed through 0.45 µm Nylon syringe filters into 2 mL HPLC autosampler vials. Where necessary, the extraction solvent was used to prepare 1:9 v/v dilutions of these extracts, so that cannabinoids with high concentrations (15–150 mg/g) could be analyzed in the same batch as those at low concentrations (0.1–15 mg/g). Both the undiluted and the diluted extract solutions were analyzed.
Medicinal cannabis oil (Cannimed; Saskatoon, Saskatchewan, Canada) was supplied by Health House International (Perth, WA, AU). The claimed composition was 10 mg/g total THC and 10 mg/g total CBD. These totals are corrected for the mass loss due to decarboxylation to report the concentrations in terms of neutral cannabinoid equivalents: CBD Total (mg/g) = CBD (mg/g) + 0.877 × CBDA (mg/g); and THC Total (mg/g) = Δ9-THC (mg/g) + 0.877 × THCA-A (mg/g). Oil samples were prepared for analysis by dissolving 50 µg in dichloromethane (1 mL), which made it miscible with the extraction solvent (total, 25 mL), and was subsequently sonicated on low power for 10 minutes. Extracts were passed through 0.45 µm Nylon syringe filters before analysis.
Instrumentation and analytical method
A Shimadzu Prominence-i LC-2030C 3D Plus HPLC-PDA (Rydalmere, NSW, AU)—comprising of a low-pressure quaternary solvent system, an auto sampler, and a PDA detector—was used. Shimadzu LabSolutions (v5.93A) was used for instrument control, data acquisition, and processing. A Phenomenex Luna C18(2) (150 × 4.6 mm × 5 µm) analytical column with a Security C18 (20 × 4.6 mm × 5 µm) guard column (Lane Cove West, NSW, AU) was employed to achieve reversed phase separation. The column was maintained at 40 °C, with a mobile phase flow rate of 2.5 mL/min. The injection volume was 10 µL, and all standards and samples were injected in duplicate. Gradient elution employing mobile phase A (Milli-Q water buffered with 20 mM ammonium formate and 0.1% formic acid), mobile phase B (acetonitrile) and mobile phase C (methanol buffered with 10 mM ammonium formate and 0.05% formic acid) was used. The gradient program is summarized in Table 1, which includes two minutes of column rinse with the organic phases and two minutes of re-equilibration at the starting condition. During each run, the PDA was set to acquire data from 190 to 800 nm and the chromatograms was visualized at 232 nm.
| ||||||||||||||||||||||||||||||||||||
System suitability
System suitability criteria were established to routinely ensure that the chromatographic system functioned as specified for each batch. To this end, a minimum of six injections of the CBD and CBDA 25 µg/mL working standard was made throughout each batch. To pass, the coefficient of variation (CV) of the standard retention times and peak areas of the six injections must be <2%.
Analysis by relative retention times and relative response factors
Standard values for the RRT and RRF were determined from three independent analysts who each performed six replicate injections of the mixed cannabinoid at the working standard concentration. The reference standards were CBD for neutral cannabinoids and CBDA for acidic cannabinoids. Adjusted retention time (tR' ) is the difference between the analyte retention time (tR) and the void time (tvoid); tR' = tR - tvoid. The RRT of a generic cannabinoid denoted as "a," relative to the reference, is the ratio of their adjusted retention times; tR' (a) / tR' (reference). Likewise, the response factor (RF) is the cannabinoid peak area (A) divided by its concentration (C); RF = A / C. The RRF of a generic cannabinoid denoted as "a," relative to the reference, is the ratio of their response factors; RF(a) / RF(reference).
Quantitative analyses using RRT and RRF values required that the CBD and CBDA working standards be tested in every batch of analysis. Using the reference retention times and the known RRT values, the expected retention time for cannabinoid "a" is determined; tR (a, expected) = [tR (reference, standard) - t0] x RRT (a) + t0. UV-vis spectra can verify this peak identification. Subsequently, the unknown concentration of cannabinoid "a" can be calculated; C(a) = [A (a, sample) / A (reference, standard)] x [1 / RRF (a)]. This represents the x C (reference, standard) concentration in the extract solution which, using the precise mass extracted in the known volumetric flask, is converted into the concentration of the original sample; reported as the mass of the cannabinoid (mg or µg) relative to the mass of the air-dried inflorescence or oil sample (/g).
Method validation
Analytical method validation was informed by the International Conference on Harmonization (ICH). [30] For the dried inflorescence sample, validation was performed for the quantification of all 10 cannabinoids. However, for the oil matrix, validation was only performed for the cannabinoids present in the preparation, namely Δ9-THC, THCA-A, CBD, and CBDA.
Linearity of the detector response was evaluated from calibration curves, considering both the R2 and the significance of the intercept. The detection and quantification limits were determined as limit of detection (LoD) = 3.3 s / m and LoQ = 10 s / m, respectively; where s is the sample standard deviation of the lowest linear concentration (3.1 µg/mL), and m is the calibration slope. The instrument and method precision were determined as the CV from six replicate standard injections and sample preparations, respectively. Intermediate precision was evaluated from the pooled CV between three analysts who independently prepared the same samples six times on separate days. The accuracy of the method was determined by a recovery study (see the "Accuracy protocol" sub-section, below). The stability of the standard and sample extracts was tested for up to 24 hours and 48 hours, respectively. Finally, the quantitative results obtained using the multipoint calibration curve and RRF method were compared.
Accuracy protocol
Recoveries of the 10 cannabinoids were tested using chamomile as a surrogate matrix for the cannabis inflorescence. For the cannabis oil, the recoveries of Δ9-THC, THCA-A, CBD, and CBDA were tested using olive oil as a surrogate matrix. These surrogate matrices were extracted according to the "Sample preparation" sub-section (above) to confirm that they did not give rise to peaks at the retention times of interest. Triplicate preparations of the surrogate matrices were spiked with the individual cannabinoid standards at levels relative to a representative sample for each matrix: Δ9-THC, CBD, and CBGA were spiked at 50%, 100%, and 200% levels; THCA-A, CBDA, and CBG were spiked at the 100% level due to limited supply; and all other cannabinoids were spiked at their LoQ.
Results and discussion
Method development
To optimize sample preparation, a variety of extraction solvents were tested with duplicate extractions. The solvents trailed were methanol, ethanol, acetonitrile, ethyl acetate, methanol:water (1:1 v/v), and acetonitrile:methanol (4:1 v/v). Cannabinoid peak areas were maximized by ethyl acetate and acetonitrile:methanol. However, due to markedly mismatching the initial mobile phase condition, ethyl acetate gave rise to significant band broadening. Thus, acetonitrile:methanol (4:1 v/v) was selected as the extraction solvent, which is consistent with other extraction optimization reports. [25,31]
The CV contribution of the method preparation procedure to the total uncertainty was determined by performing six replicate extractions and analysis of a single cannabis inflorescence sample. Grinding the inflorescence to pass through a < 710 µm sieve before subsampling achieved a CV range of 1.2 to 3.6%. When subsampling without grinding, the CV unacceptably ranged from 7.6 to 23.6%, thus indicating the importance of preparing a homogeneous sample.
To optimize the chromatographic conditions, the method was iteratively developed. Baseline separation was achieved for eight of the 10 cannabinoid standards (resolution > 2.0); however, the CBD and CBG standard peaks overlapped slightly (resolution > 1.5, in all batches), as shown in Fig. 1A. Likewise, acceptable separation of cannabinoids in the extracts of cannabis inflorescence and cannabis oil were demonstrated in Fig. 1B and C, respectively. Whilst most matrix components eluted before the cannabinoids, a compound in inflorescence samples was observed to elute between CBDA and CBGA. This peak was identified to be tetrahydrocannabivarin (THCV, tR 13.70 minutes) by comparison with the UV spectrum and retention time obtained for the THCV standard. THCV was not included in the present method validation study as it was not part of the original selected set of analytes. When the analytes were sufficiently abundant in the sample, the UV spectra of their peaks were compared to that of the standard. As shown in Fig. 2, spectra superimposed closely, indicating good peak purity.
|
|
To optimise PDA detection, wavelengths corresponding to the λmax of the different cannabinoids, specifically 210, 232, and 270 nm, were considered. Whilst 210 nm has been used in other studies [24,25,32], it produced a sloping baseline in the present study due to the use of methanol (UV cut-off 210 nm) rather than exclusively using acetonitrile (UV cut-off 190 nm) as the organic component of the mobile phase. Instead, it was found that visualizing the chromatogram at 232 nm gave the best compromise between sensitivity and baseline noise. Some studies used 270 nm to improve sensitivity for the acidic cannabinoids [33], but this higher sensitivity is not required due to their relatively high abundance in the inflorescence samples. This high abundance was anticipated as acidic cannabinoids are the secondary metabolites synthesized in cannabis, whereas the neutral forms are produced by spontaneous decarboxylation. [34]
Linearity and calibration range
Considering the seven mixed-standard dilutions prepared over the 1.6 to 250 µg/mL range, replicates at the lowest concentration for most cannabinoids deviated from their mean by >5%, indicating significant baseline noise at this level. Accordingly, calibration curves were constructed using the six standards from 3.1 to 250 µg/mL. The corresponding linear equations and their R2 are summarized in Table 2. Good linearity was obtained, with R2 > 0.9999 for all the analytes. The magnitudes of the intercepts were compared to the integrations at the working standard concentration, and all were deemed insignificant, as 3% of the working standard integration was greater than the absolute value of the intercept. Thus, the calibration equations were linear and passed sufficiently close to the origin for RRF values determined from the working standard concentration to be reliable.
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added.