Difference between revisions of "Journal:Fertilization following pollination predominantly decreases phytocannabinoids accumulation and alters the accumulation of terpenoids in Cannabis inflorescences"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 77: | Line 77: | ||
[[File:Fig1 Feder FrontPlantSci2021 12.jpg| | [[File:Fig1 Feder FrontPlantSci2021 12.jpg|1000px]] | ||
{{clear}} | {{clear}} | ||
{| | {| | ||
| STYLE="vertical-align:top;"| | | STYLE="vertical-align:top;"| | ||
{| border="0" cellpadding="5" cellspacing="0" width=" | {| border="0" cellpadding="5" cellspacing="0" width="1000px" | ||
|- | |- | ||
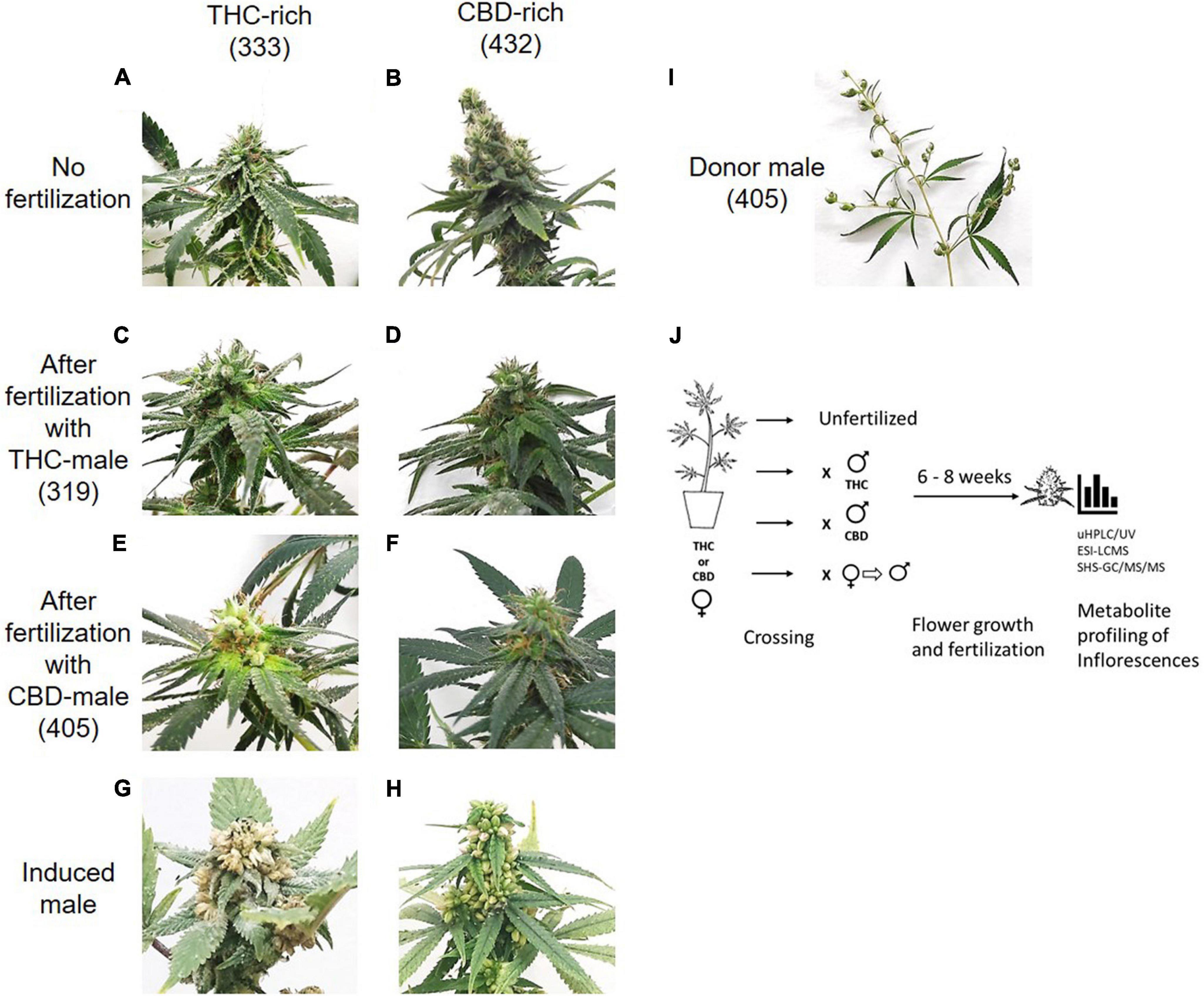
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Fig. 1''' | | style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Fig. 1''' Study design. Differences in inflorescences between THC-rich and CBD-rich plants without ('''A''', '''B''') and after fertilization with either THC-rich male (strain 319) ('''C''', '''D''') or CBD-rich male (strain 405) ('''E''', '''F''') and their respective induced-male plants ('''G''', '''H'''). ('''I''') Representative image of a male donor plant (strain 405). To capture images, the plants were placed on the same white background and photographed individually. ('''J''') Experimental design. Female ''Cannabis'' plants of two distinct types, THC- or CBD-rich chemovars, were subjected to fertilization by three different male ''Cannabis'' plants: THC- or CBD-rich plants, and an induced-male plant achieved by application of ethylene inhibitor. Female and male plants were incubated together for six to eight weeks. The profile of their secondary metabolites was analyzed by UHPLC/UV and ESI-LC/MS for phytocannabinoids and SHS-GC/MS/MS for terpenoids.</blockquote> | ||
|- | |- | ||
|} | |} | ||
|} | |} | ||
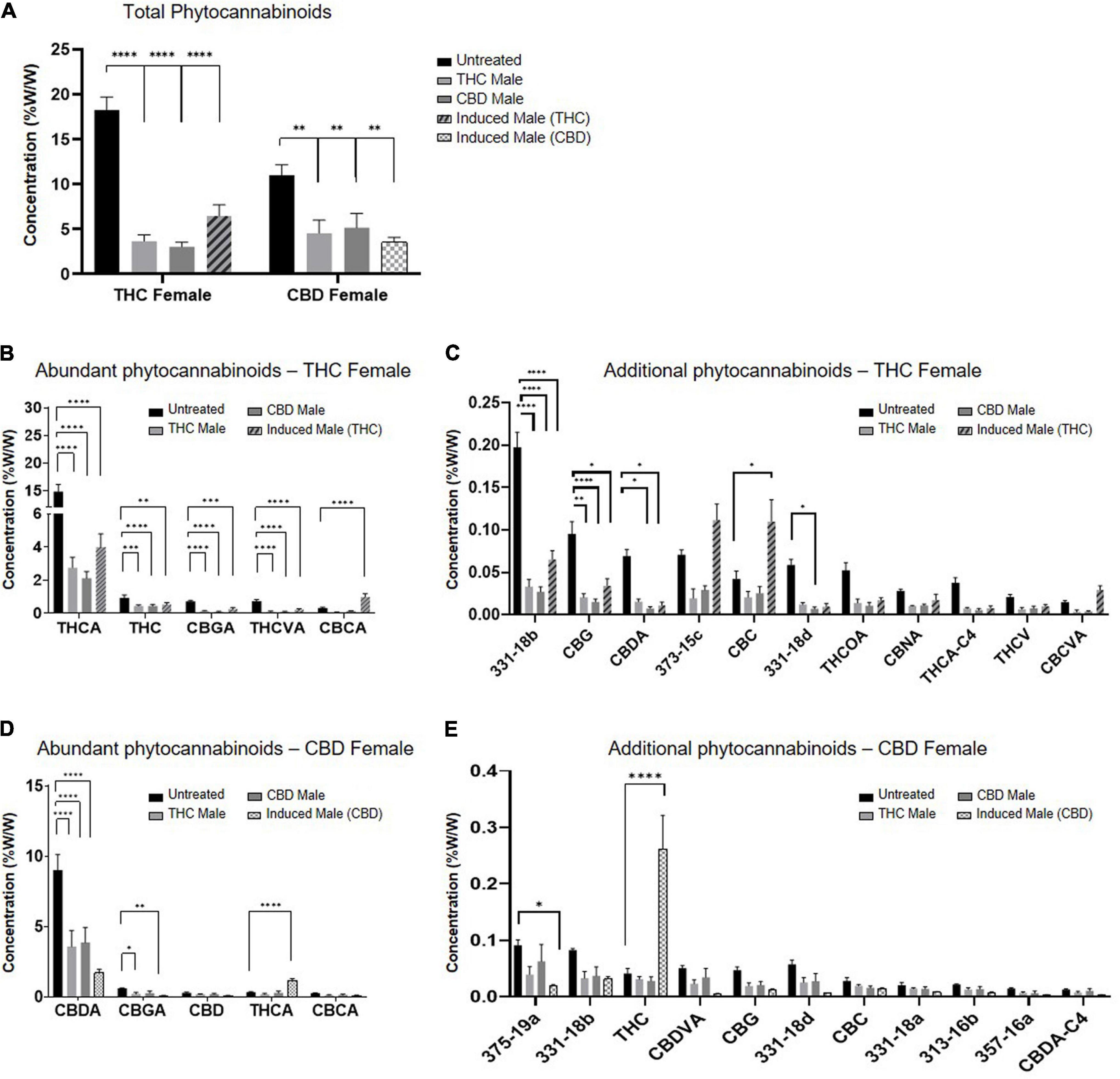
Fertilization resulted in a predominantly significant decrease of overall total phytocannabinoids concentration in inflorescences for both the THC-rich and CBD-rich females, by all three types of males (Figure 2A). The concentration of the phytocannabinoids was analyzed by UHPLC/UV and ESI-LC/MS. (The full list of the 95 phytocannabinoids quantified, as named by Berman ''et al.''<ref name=":1" />, is displayed in Supplementary material, Table 1.) A sharper decrease was detected in the THC-rich chemovar female, exhibiting an average 75% decrease, while CBD-rich females showed a 60% decrease in phytocannabinoid contents after fertilization. Next, we investigated changes in quantities of individual phytocannabinoids (Figures 2B–E). For the THC-female, fertilization caused a reduction in the abundant phytocannabinoids, whose concentrations in the plant were above 0.02%, except for the phytocannabinoid CBCA, which had an increase of about 50% when the plant was fertilized with an induced male (Figure 2B). Additional phytocannabinoids, whose concentrations in the plant were 0.001–0.2%, were also mostly reduced upon fertilization. The concentrations of CBC, cannabichromevarinic acid (CBCVA), and 373-15c were increased when fertilized by the induced male (Figure 2C). When THCA was excluded as an outlier, as its concentration is 15-fold higher, the less abundant phytocannabinoids 331-18b, CBG, CBDA, and 331-18d were significantly reduced upon fertilization. Similarly, for the CBD-female, fertilization caused a reduction in both the abundant (Figure 2D) and additional phytocannabinoids (when CBDA is excluded as an outlier) (Figure 2E), except for the concentrations of THCA and THC that increased after fertilization with the induced male. | |||
[[File:Fig2 Feder FrontPlantSci2021 12.jpg|1000px]] | |||
{{clear}} | |||
{| | |||
| STYLE="vertical-align:top;"| | |||
{| border="0" cellpadding="5" cellspacing="0" width="1000px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Fig. 2''' Phytocannabinoids quantity predominantly decreases after fertilization with all types of males. ('''A''') Total phytocannabinoid concentrations and ('''B'''–'''E''') Individual phytocannabinoid concentrations after fertilization relative to unfertilized control. Abundant phytocannabinoid concentrations were considered > 0.2% ('''B''', '''D''') and additional phytocannabinoid concentrations were 0.001–0.2% ('''C''', '''E''') in the unfertilized plants. Data are presented as mean ± SEM (''n'' = 4–6, %w/w) and statistically analyzed by two-way ANOVA followed by Dunnett’s multiple comparison test (*''p'' ≤ 0.05; **''p'' ≤ 0.01; ***''p'' ≤ 0.001; ****''p'' ≤ 0.0001). Significance in '''C''' and '''E''' was calculated after excluding THCA and CBDA, respectively, from the data.</blockquote> | |||
|- | |||
|} | |||
|} | |||
==References== | ==References== | ||
Revision as of 20:19, 26 November 2021
| Full article title | Fertilization following pollination predominantly decreases phytocannabinoids accumulation and alters the accumulation of terpenoids in Cannabis inflorescences |
|---|---|
| Journal | Frontiers in Plant Science |
| Author(s) | Feder, Carni L.; Cohen, Oded; Shapira, Anna; Katzir, Itay; Peer, Reut; Guberman, Ohad; Procaccia, Shiri; Berman, Paula; Flaishman, Moshe; Meiri, David |
| Author affiliation(s) | Technion-Israel Institute of Technology, Agricultural Research Organization of Israel |
| Primary contact | Email: dmeiri at technion dot ac dot il |
| Editors | Taglialatela-Scafati, Orazio |
| Year published | 2021 |
| Volume and issue | 12 |
| Article # | 753847 |
| DOI | 10.3389/fpls.2021.753847 |
| ISSN | 1664-462X |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.frontiersin.org/articles/10.3389/fpls.2021.753847/full |
| Download | https://www.frontiersin.org/articles/10.3389/fpls.2021.753847/pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Over the last few decades, a growing body of evidence has increasingly showed the therapeutic capabilities of Cannabis plants. These capabilities have been attributed to the specialized secondary metabolites stored in the glandular trichomes of female inflorescences, mainly phytocannabinoids and terpenoids. The accumulation of these metabolites in the flower is versatile and influenced by a largely unknown regulation system, attributed to genetic, developmental, and environmental factors. As Cannabis is a dioecious plant, one main factor is fertilization after successful pollination. Fertilized flowers are considerably less potent, likely due to changes in the contents of phytocannabinoids and terpenoids.
This study examined the effect of fertilization on metabolite composition by crossbreeding Δ9-tetrahydrocannabinol (Δ9-THC)- or cannabidiol (CBD)-rich female plants with different male plants: THC-rich plants, CBD-rich plants, or the original female plant induced to develop male pollen sacs. We used advanced analytical methods to assess the phytocannabinoid and terpenoid content, including a newly developed semi-quantitative analysis for terpenoids without analytical standards.
We found that fertilization significantly decreased phytocannabinoid content. For terpenoids, the subgroup of monoterpenoids had similar trends to the phytocannabinoids, proposing both are commonly regulated in the plant. The sesquiterpenoids remained unchanged in the THC-rich female plants and had a trend of decreasing in the CBD-rich female plants. Additionally, specific phytocannabinoids and terpenoids showed an uncommon increase in concentration following fertilization with particular male plants.
Our results demonstrate that although the profile of phytocannabinoids and their relative ratios were kept, fertilization substantially decreased the concentration of nearly all phytocannabinoids in the plant regardless of the type of fertilizing male plant. Our findings may point to the functional roles of secondary metabolites in Cannabis.
Keywords: Cannabis, cannabinoids, terpenoids, secondary metabolites, chromatography/mass spectrometry, analytical methods, gas chromatography, high pressure liquid chromatography
Introduction
Cannabis sativa L. (Cannabis) has been known as a medicinal plant since ancient times.[1] During the last two decades, many studies have added to the growing evidence for its therapeutic effects in a wide range of conditions such as neurodegenerative disorders[2][3], pain[4], epilepsy,[5] multiple sclerosis[6], and more.[7] These therapeutic abilities have been attributed to the secondary metabolites biosynthesized in Cannabis[8], with more than 500 different secondary metabolites having been identified.[9][10] These metabolites belong to several groups of compounds, including phytocannabinoids, terpenoids, and flavonoids.
To date, the most characterized are phytocannabinoids, lipophilic compounds made of isoprene units (five-carbon building blocks) (Hanuš et al., 2016), which are almost exclusive to the Cannabis plant. (Gülck and Møller, 2020) More than 140 different phytocannabinoids have been found to accumulate to various extents in glandular trichomes that are located in the aerial parts of the plant and mostly on the female flowers, which are arranged in a cluster on the stem of the inflorescence.[11] Phytocannabinoids can be classified into several subclasses according to their chemical structure, including the Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) families, as well as cannabinol (CBN), cannabigerol (CBG), cannabichromene (CBC), and many others.[10][12]
Terpenoids, found in many other plants, represent a second large group of metabolites. These metabolites are closely related to phytocannabinoids, sharing the same isoprenoid precursor and built up by branched isoprene units.[13] Terpenoids are responsible for the fragrance and taste of the plant and are suggested to also have defensive roles. They may also contribute to the therapeutic effects attributed to Cannabis.[14]
Another group of metabolites worth mentioning is flavonoids. Among this group, which is widespread in the plant kingdom, there are three specific prenylated flavonoids, termed Cannflavins A, B, and C, which are unique to Cannabis and show potent anti-inflammatory abilities.[15][16][17]
Ongoing research is focused on matching specific metabolites found in the plant and their therapeutic capabilities. To this end, specialized analytical methods have been developed in order to obtain precise knowledge on all the components of the plant and the effects they are responsible for. Currently, more than 90 phytocannabinoids and 100 terpenoids are routinely identified and quantified to obtain an overall chemical profile of each chemovar used for a medicinal purpose.[12][18] In parallel to the search for specific biological activities of the secondary metabolites, broad ongoing research is focused upon the elucidation of in planta metabolites’ biosynthesis, transport, and accumulation pathways. Genome, transcriptome, and proteome data have been published since 2011[19][20][21][22][23], and have been integrated into a genomic database for Cannabis (CannabisGDB).[24] Biosynthetic pathways are being unraveled, and recently more than 30 Cannabis-specific terpenoid synthases have been characterized.[13][22][25][26][27] In addition, the environmental and developmental factors that affect metabolite accumulation are also studied, such as light levels[28][29][30], soil types, and harvest time.[31][32][33] The increasing information on the impact of these different factors on metabolite accumulation has the prospect of developing specific chemovars harboring a pre-planned group of metabolites.[34]
This study examined the effect of an additional factor, the fertilization of Cannabis flowers following pollination of the pistil. Fertilization of flowers is a key step in the plant life cycle. Successful pollination activates a series of events followed by fertilization and embryogenesis. This includes the development of an ovary on one hand, together with senescence and abscission of floral organs, degradation of macromolecules, and recycling of different nutrients on the other hand.[35][36][37] Cannabis is a dioecious plant, harboring either female or male reproductive organs. It is also a wind-pollinated plant, in which the pollination of flowers is not dependent on specific animal pollinators. Phytocannabinoids are most abundant in the female flower inflorescences.[10] Fertilized flowers, harboring seeds, are considerably less potent. Hence the term “sinsemilla,” Spanish for “without seed,” that defines plants associated with high psychoactive effects.[38] In addition, it is a common work practice by Cannabis growers to eliminate male plants growing in a field to maintain the unfertilized inflorescences and maximize the phytocannabinoid concentrations. Therefore, it is likely that the content of secondary metabolites such as phytocannabinoids and terpenoids changes following the pollination and fertilization of Cannabis inflorescences. However, although mentioned in a few studies[14][31][39], this phenomenon was not studied in depth. In the last few years, an increasing number of Cannabis growers are moving from using cuttings from female “mother plants” to seeds. Even though the seeds are usually feminized, around 5–10% will be males, and thus the question about the effect of pollination on the phytocannabinoids and terpenoids expression becomes critical.
In order to gain more insight into the Cannabis metabolite regulation pathway, this work studied the effect of flower fertilization on the plant’s secondary metabolite accumulation. We used indoor growing methods together with analytical procedures in order to investigate the effect of fertilization on metabolite composition and concentration in Cannabis inflorescences, and specify which metabolites are affected and to what extent.
Materials and methods
Chemicals and reagents
Liquid chromatography–mass spectrometry (LC/MS)-grade acetonitrile (catalog number 1.00029), methanol (1.06035), and water (1.15333); and gas chromatography (GC) headspace-grade dimethyl sulfoxide (DMSO) (1.01900) were purchased from Mercury Scientific and Industrial Products Ltd. (Rosh Haayin, Israel). Ethanol, (catalog number 052541), acetic acid (010778) and n-Hexane (091484) were obtained from BioLab Ltd. (Jerusalem, Israel). Phytocannabinoid analytical standards (>98%) CBG, THC, CBD, CBC, CBN, cannabigerolic acid (CBGA), tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), cannabinolic acid (CBNA), cannabichromenic acid (CBCA), Δ8-tetrahydrocannabinol (Δ8-THC), tetrahydrocannabivarin (THCV), cannabidivarin (CBDV), cannabidivarinic acid (CBDVA), and cannabicyclol (CBL) were purchased from Sigma-Aldrich (Rehovot, Israel); cannabichromevarin (CBCV) was purchased from Cayman Chemical (Ann Arbor, MI, United States). Terpenoid analytical standards (>95% unless stated otherwise) were purchased from Sigma-Aldrich (Rehovot, Israel); valencene (>80% pure), α- and β-curcumene (>90% pure), α-phellandrene, and sabinene were purchased from Extrasynthese (Genay, France); a mixture of n-alkanes was purchased from Sigma R 769 (40 mg/mL, C8-C20, Saint Louis, MO, United States) for semi-quantitative analysis.
Experimental design
The effect of fertilization was tested on two Cannabis sativa L. female plants. Female strains 333 THC-rich (15% THCA, 0.07% CBDA) and 423 CBD-rich (0.33% THCA, 9% CBDA) were subjected to fertilization, and two male plants strains—319 THC-rich (progeny of high THC landrace Highland Thai, Seedsman seeds) and 405 CBD-rich (progeny of Cherry CBD)—were used as pollen donors. In addition, the female plants were subjected to a sex conversion treatment[40][41], and these induced males were also used as pollen donors to fertilize the two female plants. In order to achieve pollen sacs, 45-days old rooted cutting, 30 cm size female plants were sprayed daily until completely moist with ethylene inhibitor (Sodium Thiosulfate 0.5%) for five days prior to transferring to short day conditions. The female plants that were sex changed are referred to as males or induced-males. The female Cannabis plants were grown, three plants for each treatment, under an 18/6 light/dark regime (800 μmol), 23–27°C for 30 days before being transferred to flowering chambers with a 12/12 light/dark regime (500 μmol), 23–27°C for up to either 42 (six weeks) or 56 (eight weeks) days before some inflorescences were removed for chemical analysis. Female plants were grown in small flowering chambers (1 m2) in the presence of a single pollen donor. All plants were grown in 1 L pots on a mixture of pit/coconut 70%/30% soil, respectively. l The inflorescences of the treated plants, averaging 3–4 apical inflorescences per plant, were harvested and dried for 24–48 hours at 40°C until they reached a moisture content of 12% weight for weight (w/w). The inflorescences were ground to a fine powder using an electric grinder, then 98–103 mg were weighed and extracted with 1 mL ethanol. Samples were sonicated in an ultrasonic bath for 30 minutes, agitated in an orbital shaker at 25°C for 20 minutes, centrifuged at 20,000 x g for 5 minutes, then the samples were dissolved and diluted x20 in ethanol and filtered through a 0.22 μm Polytetrafluoroethylene syringe filter (Lumitron Ltd., Petah Tikva, Israel) prior to analysis.
Phytocannabinoid identification and quantification
Phytocannabinoid analyses for high concentrations of THC and CBD were performed using a Thermo Scientific UltiMate 3000 ultra-high-performance liquid chromatography coupled with an ultraviolet-visible diode array detector (UHPLC/UV) system. All other phytocannabinoids were identified and quantified by a similar UHPLC instrument coupled with a Q Exactive Focus Hybrid Quadrupole-Orbitrap MS (Thermo Scientific, Bremen, Germany), as previously described by Berman et al.[12] and Milay et al.[42] In short, chromatographic separation was achieved using a HALO C18 Fused-Core column (2.7 μm, 150 × 2.1 mm), with a HALO guard column (2.7 μm, 5 × 2.1 mm), and a ternary A/B/C multistep gradient (solvent A: water with 0.1% acetic acid; solvent B: acetonitrile with 0.1% acetic acid,; and solvent C: methanol). Identification and absolute quantification of phytocannabinoids were performed by external calibrations, as previously described by Berman et al.[12] Sixteen analytical standards (CBDVA, CBDA, CBCA, CBNA, CBGA, THCA, CBDV, CBD, CBC, CBN, CBG, THC, Δ8-THC, CBL, THCV, CBCV) were used for direct quantification and semi-quantification of additional phytocannabinoids. All extracted samples were injected and analyzed by an electrospray ionization (ESI)-LC/MS analysis, diluted at ratios of 1:9, 1:99, and 1:999 v/v Cannabis extract to ethanol.
Terpenoids identification and quantification
Profiling of terpenoids was performed using a modification of the static headspace gas chromatography–tandem mass spectrometry (SHS-GC/MS/MS) method by full evaporation technique.[18] SHS-injections were performed by PAL RTC robotic tool (CTC Analytics, Swaziland) with 30 minute incubation time, temperature of 140°C, and 1,000 μL injection volume of the gas phase. Gas chromatographic separation was achieved in 74 minutes using a TRACE 1310 GC (Thermo Fisher Scientific, Bremen, Germany) equipped with a 30 m × 0.25 mm × 0.25 μm capillary DB-35MS UI column (Agilent Technologies, United States). MS/MS compound detection was performed by a TSQ 8000 Evo triple quadrupole mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). For terpenoids analyses, 10 mg of each ground Cannabis sample was weighed in duplicates in a 20 mL HS amber vial with 1.2 g of glycerol and sealed by a magnetic cap. Solutions for the construction of the calibration curves were prepared in hexane, and then 10 μL for each calibration level was added to amber vials with 1.2 g of glycerol in the same manner as the samples.
Some of the terpenoids were calculated semi-quantitatively based on the calibration curves of terpenoids with commercially available analytical standards with similar MS spectral characteristics and retention times. Identification of these terpenoids was performed by spectral searching against the NIST library (version 2.2) and relative Kovats retention indices using a mixture of n-alkanes run under the same chromatographic conditions (for full details, see Supplementary material, Tables 2 and 3).
Statistical analysis
Statistical analyses were conducted using GraphPad Prism software version 8.2.1 (GraphPad Inc.). Differences between samples in phytocannabinoid and terpenoid concentrations were analyzed using two-way ANOVA followed by Dunnett’s multiple comparison test. P-values were corrected for multiple testing using the Tukey post hoc test. A value of at least p ≤ 0.05 was considered significant for all tests (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001). Outliers were defined as data points greater than two standard deviations from the mean (9.6 for THCA and 9.5 for CBDA).
Results
Phytocannabinoids quantity predominantly decreases after fertilization
Mature inflorescences (six or eight weeks post-flower-induction) from female Cannabis plants of two distinct chemovars (Figures 1A and B), THC-rich (Type I) and CBD-rich (Type III), were subjected to fertilization by three different male Cannabis plant types: THC-rich (Figures 1C and D), CBD-rich (Figures 1E and F), or the original female plant induced to develop male pollen sacs by application of ethylene inhibitor (Figure 1). Induced-male plants (Figures 1G and H) were genetically identical to the female plants, and they had a distinct change in the sex of the flowers after treatment and a larger number of inflorescences compared to males (Figure 1I). Specific fertilization was achieved by incubation of the individual plants. (Figure 1J)
|
Fertilization resulted in a predominantly significant decrease of overall total phytocannabinoids concentration in inflorescences for both the THC-rich and CBD-rich females, by all three types of males (Figure 2A). The concentration of the phytocannabinoids was analyzed by UHPLC/UV and ESI-LC/MS. (The full list of the 95 phytocannabinoids quantified, as named by Berman et al.[12], is displayed in Supplementary material, Table 1.) A sharper decrease was detected in the THC-rich chemovar female, exhibiting an average 75% decrease, while CBD-rich females showed a 60% decrease in phytocannabinoid contents after fertilization. Next, we investigated changes in quantities of individual phytocannabinoids (Figures 2B–E). For the THC-female, fertilization caused a reduction in the abundant phytocannabinoids, whose concentrations in the plant were above 0.02%, except for the phytocannabinoid CBCA, which had an increase of about 50% when the plant was fertilized with an induced male (Figure 2B). Additional phytocannabinoids, whose concentrations in the plant were 0.001–0.2%, were also mostly reduced upon fertilization. The concentrations of CBC, cannabichromevarinic acid (CBCVA), and 373-15c were increased when fertilized by the induced male (Figure 2C). When THCA was excluded as an outlier, as its concentration is 15-fold higher, the less abundant phytocannabinoids 331-18b, CBG, CBDA, and 331-18d were significantly reduced upon fertilization. Similarly, for the CBD-female, fertilization caused a reduction in both the abundant (Figure 2D) and additional phytocannabinoids (when CBDA is excluded as an outlier) (Figure 2E), except for the concentrations of THCA and THC that increased after fertilization with the induced male.
|
References
- ↑ Bonini, Sara Anna; Premoli, Marika; Tambaro, Simone; Kumar, Amit; Maccarinelli, Giuseppina; Memo, Maurizio; Mastinu, Andrea (1 December 2018). "Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history" (in en). Journal of Ethnopharmacology 227: 300–315. doi:10.1016/j.jep.2018.09.004. https://linkinghub.elsevier.com/retrieve/pii/S0378874118316611.
- ↑ Fernández-Ruiz, Javier (1 May 2019). "The biomedical challenge of neurodegenerative disorders: an opportunity for cannabinoid-based therapies to improve on the poor current therapeutic outcomes: Cannabinoids and neuroprotection" (in en). British Journal of Pharmacology 176 (10): 1370–1383. doi:10.1111/bph.14382. PMC PMC6487558. PMID 29856067. https://onlinelibrary.wiley.com/doi/10.1111/bph.14382.
- ↑ Cassano, Tommaso; Villani, Rosanna; Pace, Lorenzo; Carbone, Antonio; Bukke, Vidyasagar Naik; Orkisz, Stanislaw; Avolio, Carlo; Serviddio, Gaetano (6 March 2020). "From Cannabis sativa to Cannabidiol: Promising Therapeutic Candidate for the Treatment of Neurodegenerative Diseases". Frontiers in Pharmacology 11: 124. doi:10.3389/fphar.2020.00124. ISSN 1663-9812. PMC PMC7069528. PMID 32210795. https://www.frontiersin.org/article/10.3389/fphar.2020.00124/full.
- ↑ Starowicz, Katarzyna; Finn, David P. (2017), "Cannabinoids and Pain: Sites and Mechanisms of Action" (in en), Advances in Pharmacology (Elsevier) 80: 437–475, doi:10.1016/bs.apha.2017.05.003, ISBN 978-0-12-811232-8, https://linkinghub.elsevier.com/retrieve/pii/S1054358917300443. Retrieved 2021-11-24
- ↑ Franco, Valentina; Bialer, Meir; Perucca, Emilio (1 March 2021). "Cannabidiol in the treatment of epilepsy: Current evidence and perspectives for further research" (in en). Neuropharmacology 185: 108442. doi:10.1016/j.neuropharm.2020.108442. https://linkinghub.elsevier.com/retrieve/pii/S0028390820305104.
- ↑ Rice, Jessica; Cameron, Michelle (1 August 2018). "Cannabinoids for Treatment of MS Symptoms: State of the Evidence" (in en). Current Neurology and Neuroscience Reports 18 (8): 50. doi:10.1007/s11910-018-0859-x. ISSN 1528-4042. http://link.springer.com/10.1007/s11910-018-0859-x.
- ↑ Gonçalves, Joana; Rosado, Tiago; Soares, Sofia; Simão, Ana; Caramelo, Débora; Luís, Ângelo; Fernández, Nicolás; Barroso, Mário et al. (23 February 2019). "Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination" (in en). Medicines 6 (1): 31. doi:10.3390/medicines6010031. ISSN 2305-6320. PMC PMC6473697. PMID 30813390. http://www.mdpi.com/2305-6320/6/1/31.
- ↑ Andre, Christelle M.; Hausman, Jean-Francois; Guerriero, Gea (4 February 2016). "Cannabis sativa: The Plant of the Thousand and One Molecules". Frontiers in Plant Science 7: 19. doi:10.3389/fpls.2016.00019. ISSN 1664-462X. PMC PMC4740396. PMID 26870049. http://journal.frontiersin.org/Article/10.3389/fpls.2016.00019/abstract.
- ↑ ElSohly, Mahmoud A.; Slade, Desmond (1 December 2005). "Chemical constituents of marijuana: The complex mixture of natural cannabinoids" (in en). Life Sciences 78 (5): 539–548. doi:10.1016/j.lfs.2005.09.011. https://linkinghub.elsevier.com/retrieve/pii/S002432050500891X.
- ↑ 10.0 10.1 10.2 Flores-Sanchez, Isvett Josefina; Verpoorte, Robert (1 October 2008). "Secondary metabolism in cannabis" (in en). Phytochemistry Reviews 7 (3): 615–639. doi:10.1007/s11101-008-9094-4. ISSN 1568-7767. http://link.springer.com/10.1007/s11101-008-9094-4.
- ↑ Hanuš, Lumír Ondřej; Meyer, Stefan Martin; Muñoz, Eduardo; Taglialatela-Scafati, Orazio; Appendino, Giovanni (2016). "Phytocannabinoids: a unified critical inventory" (in en). Natural Product Reports 33 (12): 1357–1392. doi:10.1039/C6NP00074F. ISSN 0265-0568. http://xlink.rsc.org/?DOI=C6NP00074F.
- ↑ 12.0 12.1 12.2 12.3 12.4 Berman, Paula; Futoran, Kate; Lewitus, Gil M.; Mukha, Dzmitry; Benami, Maya; Shlomi, Tomer; Meiri, David (1 December 2018). "A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis" (in en). Scientific Reports 8 (1): 14280. doi:10.1038/s41598-018-32651-4. ISSN 2045-2322. PMC PMC6155167. PMID 30250104. http://www.nature.com/articles/s41598-018-32651-4.
- ↑ 13.0 13.1 Booth, Judith K.; Yuen, Macaire M.S.; Jancsik, Sharon; Madilao, Lufiani L.; Page, Jonathan E.; Bohlmann, Jörg (1 September 2020). "Terpene Synthases and Terpene Variation in Cannabis sativa" (in en). Plant Physiology 184 (1): 130–147. doi:10.1104/pp.20.00593. ISSN 0032-0889. PMC PMC7479917. PMID 32591428. https://academic.oup.com/plphys/article/184/1/130-147/6117797.
- ↑ 14.0 14.1 Russo, Ethan B.; Marcu, Jahan (2017), "Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads" (in en), Advances in Pharmacology (Elsevier) 80: 67–134, doi:10.1016/bs.apha.2017.03.004, ISBN 978-0-12-811232-8, https://linkinghub.elsevier.com/retrieve/pii/S1054358917300273. Retrieved 2021-11-24
- ↑ Radwan, Mohamed M.; ElSohly, Mahmoud A.; Slade, Desmond; Ahmed, Safwat A.; Wilson, Lisa; El-Alfy, Abir T.; Khan, Ikhlas A.; Ross, Samir A. (1 October 2008). "Non-cannabinoid constituents from a high potency Cannabis sativa variety" (in en). Phytochemistry 69 (14): 2627–2633. doi:10.1016/j.phytochem.2008.07.010. PMC PMC4888767. PMID 18774146. https://linkinghub.elsevier.com/retrieve/pii/S0031942208003518.
- ↑ Rea, Kevin A; Casaretto, José A.; Al-Abdul-Wahid, M. Sameer; Sukumaran, Arjun; Geddes-McAlister, Jennifer; Rothstein, Steven J.; Akhtar, Tariq A. (1 August 2019). "Biosynthesis of cannflavins A and B from Cannabis sativa L" (in en). Phytochemistry 164: 162–171. doi:10.1016/j.phytochem.2019.05.009. https://linkinghub.elsevier.com/retrieve/pii/S0031942218303819.
- ↑ Erridge, Simon; Mangal, Nagina; Salazar, Oliver; Pacchetti, Barbara; Sodergren, Mikael H. (1 October 2020). "Cannflavins – From plant to patient: A scoping review" (in en). Fitoterapia 146: 104712. doi:10.1016/j.fitote.2020.104712. https://linkinghub.elsevier.com/retrieve/pii/S0367326X2030294X.
- ↑ 18.0 18.1 Shapira, Anna; Berman, Paula; Futoran, Kate; Guberman, Ohad; Meiri, David (3 September 2019). "Tandem Mass Spectrometric Quantification of 93 Terpenoids in Cannabis Using Static Headspace Injections" (in en). Analytical Chemistry 91 (17): 11425–11432. doi:10.1021/acs.analchem.9b02844. ISSN 0003-2700. https://pubs.acs.org/doi/10.1021/acs.analchem.9b02844.
- ↑ van Bakel, Harm; Stout, Jake M; Cote, Atina G; Tallon, Carling M; Sharpe, Andrew G; Hughes, Timothy R; Page, Jonathan E (2011). "The draft genome and transcriptome of Cannabis sativa" (in en). Genome Biology 12 (10): R102. doi:10.1186/gb-2011-12-10-r102. ISSN 1465-6906. PMC PMC3359589. PMID 22014239. http://genomebiology.biomedcentral.com/articles/10.1186/gb-2011-12-10-r102.
- ↑ Laverty, Kaitlin U.; Stout, Jake M.; Sullivan, Mitchell J.; Shah, Hardik; Gill, Navdeep; Holbrook, Larry; Deikus, Gintaras; Sebra, Robert et al. (1 January 2019). "A physical and genetic map of Cannabis sativa identifies extensive rearrangements at the THC/CBD acid synthase loci" (in en). Genome Research 29 (1): 146–156. doi:10.1101/gr.242594.118. ISSN 1088-9051. PMC PMC6314170. PMID 30409771. http://genome.cshlp.org/lookup/doi/10.1101/gr.242594.118.
- ↑ Vincent, Delphine; Rochfort, Simone; Spangenberg, German (13 February 2019). "Optimisation of Protein Extraction from Medicinal Cannabis Mature Buds for Bottom-Up Proteomics" (in en). Molecules 24 (4): 659. doi:10.3390/molecules24040659. ISSN 1420-3049. PMC PMC6412734. PMID 30781766. http://www.mdpi.com/1420-3049/24/4/659.
- ↑ 22.0 22.1 Livingston, Samuel J.; Quilichini, Teagen D.; Booth, Judith K.; Wong, Darren C. J.; Rensing, Kim H.; Laflamme‐Yonkman, Jessica; Castellarin, Simone D.; Bohlmann, Joerg et al. (1 January 2020). "Cannabis glandular trichomes alter morphology and metabolite content during flower maturation" (in en). The Plant Journal 101 (1): 37–56. doi:10.1111/tpj.14516. ISSN 0960-7412. https://onlinelibrary.wiley.com/doi/10.1111/tpj.14516.
- ↑ McGarvey, Peter; Huang, Jiahao; McCoy, Matthew; Orvis, Joshua; Katsir, Yael; Lotringer, Nitzan; Nesher, Iris; Kavarana, Malcolm et al. (1 December 2020). "De novo assembly and annotation of transcriptomes from two cultivars of Cannabis sativa with different cannabinoid profiles" (in en). Gene 762: 145026. doi:10.1016/j.gene.2020.145026. https://linkinghub.elsevier.com/retrieve/pii/S0378111920306958.
- ↑ Cai, Sen; Zhang, Zhiyuan; Huang, Suyun; Bai, Xu; Huang, Ziying; Zhang, Yiping Jason; Huang, Likun; Tang, Weiqi et al. (1 May 2021). "CannabisGDB: a comprehensive genomic database for Cannabis Sativa L" (in en). Plant Biotechnology Journal 19 (5): 857–859. doi:10.1111/pbi.13548. ISSN 1467-7644. PMC PMC8131054. PMID 33462958. https://onlinelibrary.wiley.com/doi/10.1111/pbi.13548.
- ↑ Booth, Judith K.; Page, Jonathan E.; Bohlmann, Jörg (29 March 2017). Hamberger, Björn. ed. "Terpene synthases from Cannabis sativa" (in en). PLOS ONE 12 (3): e0173911. doi:10.1371/journal.pone.0173911. ISSN 1932-6203. PMC PMC5371325. PMID 28355238. https://dx.plos.org/10.1371/journal.pone.0173911.
- ↑ Allen, Keith D.; McKernan, Kevin; Pauli, Christopher; Roe, Jim; Torres, Anthony; Gaudino, Reggie (12 September 2019). Hamberger, Björn. ed. "Genomic characterization of the complete terpene synthase gene family from Cannabis sativa" (in en). PLOS ONE 14 (9): e0222363. doi:10.1371/journal.pone.0222363. ISSN 1932-6203. PMC PMC6742361. PMID 31513654. https://dx.plos.org/10.1371/journal.pone.0222363.
- ↑ Zager, Jordan J.; Lange, Iris; Srividya, Narayanan; Smith, Anthony; Lange, B. Markus (1 August 2019). "Gene Networks Underlying Cannabinoid and Terpenoid Accumulation in Cannabis" (in en). Plant Physiology 180 (4): 1877–1897. doi:10.1104/pp.18.01506. ISSN 0032-0889. PMC PMC6670104. PMID 31138625. https://academic.oup.com/plphys/article/180/4/1877-1897/6117720.
- ↑ Hawley, Dave; Graham, Thomas; Stasiak, Michael; Dixon, Mike (1 November 2018). "Improving Cannabis Bud Quality and Yield with Subcanopy Lighting". HortScience 53 (11): 1593–1599. doi:10.21273/HORTSCI13173-18. ISSN 0018-5345. https://journals.ashs.org/view/journals/hortsci/53/11/article-p1593.xml.
- ↑ Magagnini, Gianmaria; Grassi, Gianpaolo; Kotiranta, Stiina (12 June 2018). "The Effect of Light Spectrum on the Morphology and Cannabinoid Content of Cannabis sativa L." (in en). Medical Cannabis and Cannabinoids 1 (1): 19–27. doi:10.1159/000489030. ISSN 2504-3889. PMC PMC8489345. PMID 34676318. https://www.karger.com/Article/FullText/489030.
- ↑ Namdar, Dvory; Charuvi, Dana; Ajjampura, Vinayka; Mazuz, Moran; Ion, Aurel; Kamara, Itzhak; Koltai, Hinanit (1 June 2019). "LED lighting affects the composition and biological activity of Cannabis sativa secondary metabolites" (in en). Industrial Crops and Products 132: 177–185. doi:10.1016/j.indcrop.2019.02.016. https://linkinghub.elsevier.com/retrieve/pii/S0926669019301086.
- ↑ 31.0 31.1 Meier, C.; Mediavilla, V. (1998). "Factors influencing the yield and the quality of hemp (Cannabis sativa L.) essential oil". Journal of the International Hemp Association 5 (1): 16–20. http://www.internationalhempassociation.org/jiha/jiha5107.html.
- ↑ Bernstein, Nirit; Gorelick, Jonathan; Zerahia, Roei; Koch, Sraya (17 June 2019). "Impact of N, P, K, and Humic Acid Supplementation on the Chemical Profile of Medical Cannabis (Cannabis sativa L)". Frontiers in Plant Science 10: 736. doi:10.3389/fpls.2019.00736. ISSN 1664-462X. PMC PMC6589925. PMID 31263470. https://www.frontiersin.org/article/10.3389/fpls.2019.00736/full.
- ↑ Chandra, Suman; Lata, Hemant; ElSohly, Mahmoud A. (26 June 2020). "Propagation of Cannabis for Clinical Research: An Approach Towards a Modern Herbal Medicinal Products Development". Frontiers in Plant Science 11: 958. doi:10.3389/fpls.2020.00958. ISSN 1664-462X. PMC PMC7333344. PMID 32676092. https://www.frontiersin.org/article/10.3389/fpls.2020.00958/full.
- ↑ Romero, P.; Peris, A.; Vergara, K.; Matus, J.T. (1 September 2020). "Comprehending and improving cannabis specialized metabolism in the systems biology era" (in en). Plant Science 298: 110571. doi:10.1016/j.plantsci.2020.110571. https://linkinghub.elsevier.com/retrieve/pii/S0168945220301771.
- ↑ O'Neill, Sharman D. (1 June 1997). "POLLINATION REGULATION OF FLOWER DEVELOPMENT" (in en). Annual Review of Plant Physiology and Plant Molecular Biology 48 (1): 547–574. doi:10.1146/annurev.arplant.48.1.547. ISSN 1040-2519. https://www.annualreviews.org/doi/10.1146/annurev.arplant.48.1.547.
- ↑ Tripathi, Siddharth Kaushal; Tuteja, Narendra (1 November 2007). "Integrated Signaling in Flower Senescence: An Overview" (in en). Plant Signaling & Behavior 2 (6): 437–445. doi:10.4161/psb.2.6.4991. ISSN 1559-2324. PMC PMC2634333. PMID 19517004. http://www.tandfonline.com/doi/abs/10.4161/psb.2.6.4991.
- ↑ Borghi, Monica; Fernie, Alisdair R. (1 August 2020). "Outstanding questions in flower metabolism" (in en). The Plant Journal 103 (4): 1275–1288. doi:10.1111/tpj.14814. ISSN 0960-7412. https://onlinelibrary.wiley.com/doi/10.1111/tpj.14814.
- ↑ Potter, David J.; Hammond, Kathy; Tuffnell, Shaun; Walker, Christopher; Di Forti, Marta (1 April 2018). "Potency of Δ 9 -tetrahydrocannabinol and other cannabinoids in cannabis in England in 2016: Implications for public health and pharmacology" (in en). Drug Testing and Analysis 10 (4): 628–635. doi:10.1002/dta.2368. https://onlinelibrary.wiley.com/doi/10.1002/dta.2368.
- ↑ Potter, D. (March 2009). "The Propagation, Characterisation and Optimisation of Cannabis sativa L. as a Phytopharmaceutical" (PDF). King’s College London. https://extractionmagazine.com/wp-content/uploads/2018/06/THE-PROPAGATION-CHARACTERISATION-AND-OPTIMISATION-OF-CANNABIS-SATIVA-L-AS-A-PHYTOPHARMACEUTICAL.pdf.
- ↑ Mohan Ram, H. Y.; Sett, R. (1 December 1982). "Induction of fertile male flowers in genetically female Cannabis sativa plants by silver nitrate and silver thiosulphate anionic complex" (in en). Theoretical and Applied Genetics 62 (4): 369–375. doi:10.1007/BF00275107. ISSN 0040-5752. http://link.springer.com/10.1007/BF00275107.
- ↑ Small, Ernest; Naraine, Steve G. U. (1 February 2016). "Expansion of female sex organs in response to prolonged virginity in Cannabis sativa (marijuana)" (in en). Genetic Resources and Crop Evolution 63 (2): 339–348. doi:10.1007/s10722-015-0253-3. ISSN 0925-9864. http://link.springer.com/10.1007/s10722-015-0253-3.
- ↑ Milay, Looz; Berman, Paula; Shapira, Anna; Guberman, Ohad; Meiri, David (15 October 2020). "Metabolic Profiling of Cannabis Secondary Metabolites for Evaluation of Optimal Postharvest Storage Conditions". Frontiers in Plant Science 11: 583605. doi:10.3389/fpls.2020.583605. ISSN 1664-462X. PMC PMC7593247. PMID 33178249. https://www.frontiersin.org/article/10.3389/fpls.2020.583605/full.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. The original article lists references in alphabetical order; however, this version lists them in order of appearance, by design.