Journal:Does cannabis extract obtained from cannabis flowers with maximum allowed residual level of aflatoxins and ochratoxin A have an impact on human safety and health?
| Full article title | Does cannabis extract obtained from cannabis flowers with maximum allowed residual level of aflatoxins and ochratoxin A have an impact on human safety and health? |
|---|---|
| Journal | Frontiers in Medicine |
| Author(s) | Serafimovska, Tijana; Stefanovski, Sasho; Erler, Joachim; Keskovski, Zlatko; Stefkov, Gjoshe; Mitevska, Marija; Serafimovska, Marija D.; Balkanov, Trajan; Ribarska, Jasmina T. |
| Author affiliation(s) | Ss. Cyril and Methodius University of Skopje, NYSK Holdings, Diapharm GmbH & Co. KG, Goce Delchev University |
| Primary contact | Email: serafimovskatijana at gmail dot com |
| Editors | Bolcato, Matteo |
| Year published | 2021 |
| Volume and issue | 8 |
| Article # | 759856 |
| DOI | 10.3389/fmed.2021.759856 |
| ISSN | 2296-858X |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.frontiersin.org/articles/10.3389/fmed.2021.759856/full |
| Download | https://www.frontiersin.org/articles/10.3389/fmed.2021.759856/pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Aim: The aim of this study was to investigate whether the cannabis extract obtained from cannabis flowers that contain the maximum allowed level of mycotoxins affects human safety and health. For that purpose, a novel method of liquid chromatography with tandem mass spectrometry (LC-MS/MS) was developed and validated for the determination of aflatoxins and ochratoxin A (OchA) in cannabis extracts to demonstrate that this analytical method is suitable for the intended experimental design.
Methods: Experimental design was done by adding maximum allowed concentration of aflatoxins (B1, B2, G1, G2) and OchA according to the European Pharmacopeia related to cannabis flowers. The concentration of aflatoxins and OchA was determined using the same LC-MS/MS analytical method on the initial plant material (dry flower) before preparing the spiked sample and after obtaining decarboxylated extract with ethanol 96%.
Results: The results obtained indicate that aflatoxins and OchA, primarily added to the cannabis dried flowers, were also determined into the obtained final extract in amounts much higher (m/m) than in the initial plant material.
Conclusion: With this experiment, we have shown that mycotoxins, especially aflatoxins, which are extremely toxic secondary metabolites, can reach critical values in cannabis extracts obtained from dry cannabis flowers with the maximum allowed quantity of mycotoxins. This can pose a great risk to consumers and their health, especially to those with compromised immune systems.
Keywords: mycotoxins, aflatoxins, ochratoxin A, determination liquid chromatography with tandem mass spectrometry (LC/MS/MS), cannabis extracts
Introduction
In traditional medicine, medicinal products based on Cannabis sativa L. (Cannabaceae) have been used for thousands of years in the treatment of various diseases. [1] Although, there is a lack of evidence-based medical information that can prove the potential benefit of the therapy with medicinal cannabis preparations, recently, an increasing number of pharmacists have issued cannabis preparations to individual patients prescribed by their physicians. [2]
To obtain cannabis-based preparations, standardized concentrated cannabinoid extracts, produced by a suitable extraction process of cannabinoids from cannabis flowers, are used. [2] The safety of the flowers as a starting material, in this case, is of the utmost significance for human safety and health. Since there is no monograph in the European Pharmacopeia for quality testing of cannabis flowers, currently a revised monograph for cannabis flower (cannabis floss), published by the German Federal Institute for Drugs and Medical Devices (BfArM) in the German Pharmacopoeia [3], instructs the obligatory procedure for quality testing of cannabis flowers in the European Union. [4] However, a variety of herbal monographs are listed under the general monographs in the European Pharmacopeia: herbal drugs, herbal drug extracts, and herbal drug preparations. These general monographs are created to cover products and quality parameters, which are not mentioned in the individual monographs. Therefore, it is necessary to apply the individual monograph always in combination with these quality requirements. [5]
According to the European Medicines Agency's "Guideline on specifications: test procedures and acceptance criteria for herbal substances, herbal preparations and herbal medicinal products" [6], mycotoxins (i.e., aflatoxins and ochratoxin A [OchA]) are considered impurities (contaminants) that can occur in the final extracts from their initial plant materials (herbal drugs). In reference to this, the European Pharmacopeia gives the maximum allowed limits of these impurities (aflatoxins, as per European Pharmacopeia 2.8.18, and OchA, as per European Pharmacopeia 2.8.22) in herbal drugs.
Impact of mycotoxins on human safety and health
Mycotoxins like aflatoxins and OchA are secondary toxic metabolites, obtained primarily from fungal species (Penicillium and Aspergillus). Fungi and their metabolites contaminate the raw materials usually used in the preparation of products for human use. [7] The presence of these contaminants in products for human use can cause various acute and chronic effects on human health and safety. [8]
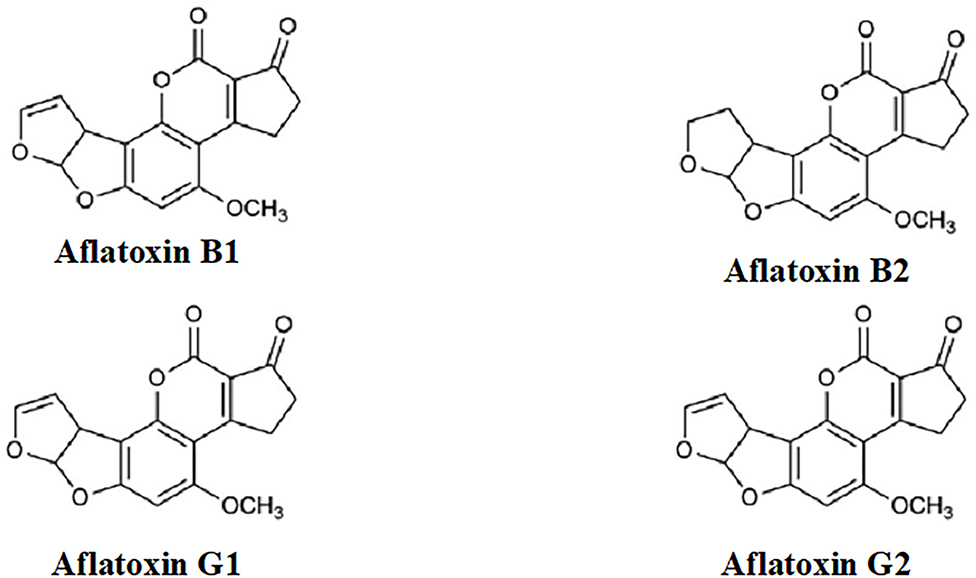
Aflatoxins (Figure 1) are extremely toxic secondary metabolites. According to their chemical structures, they are generally categorized into two groups: the difurocoumarocyclopentenone group (aflatoxin B1 and B2) and the difurocoumarolactone group (aflatoxin G1 and G2). The most toxic, carcinogenic, and mutagenic among all the aflatoxins is aflatoxin B1 (AfB1). Humans are mostly infected by direct ingestion (consuming) of infected herbal drugs, food, or herbal preparations. [9]
|
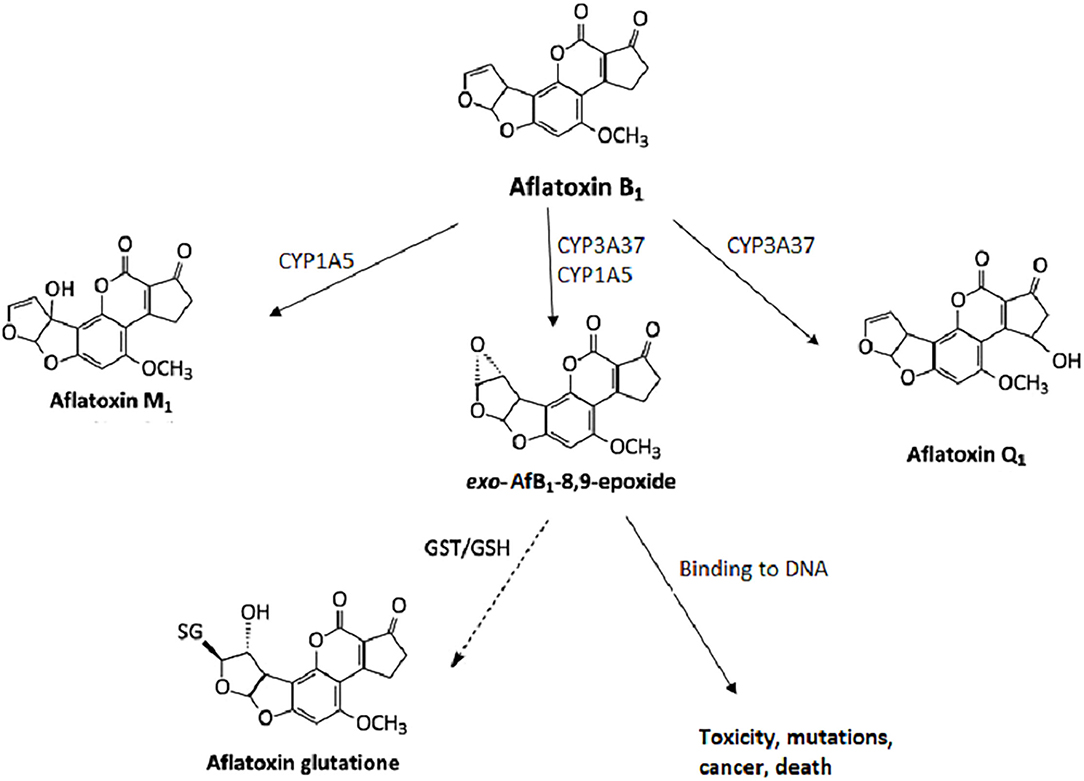
Mycotoxins are metabolized in the liver, and microorganisms in the digestive tract. [10, 11] AfB1 itself is not carcinogenic, but in the human body, it is metabolized into carcinogenic metabolites. All enzymes necessary for the bioactivation of AfB1 are present in the nuclear envelope of the hepatocytes. The mono-oxygenase system in microsomes can transform the AfB1 into aflatoxin M1 (AfM1), aflatoxin Q1 (AfQ1), and exo-AfB1-8,9-epoxide (Figure 2), which can bind to deoxyribonucleic acid (DNA) and causes cytotoxicity, DNA damage, chromosomal anomalies, gene mutation, and cell transformation by attacking the nucleophilic hetero-atoms such as oxygen and nitrogen in the organic bases of nucleic acids and forming a strong covalent bond to the DNA. [9]
|
Specific effects of aflatoxins on human safety and health can be classified into two groups: chronic toxicity and acute toxicity.
Carcinogenicity, hepatotoxicity, nephrotoxicity, and endocrine disorders have been related to chronic exposure to low levels of mycotoxins. [12, 13] In some cases, allergic reactions, immune diseases, reproductive deficiencies, fetal alterations, and death have also been related to chronic exposure to mycotoxins. [14] The health risks associated with mycotoxin exposure arise from their toxicity and depend on the type of toxin, its metabolism, the immune system, and the health status of the exposed individual. [15] Due to the carcinogenic risk associated with the mycotoxins, the International Agency for Research on Cancer (IARC) has evaluated and classified mycotoxins as carcinogenic to humans (Group 1), possibly carcinogenic to humans (Group 2B), or not classifiable as to its carcinogenicity to humans (Group 3), based on sufficient experimental data. [16] The main target organ for toxicity, mutagenicity, and carcinogenicity is the liver. [17]
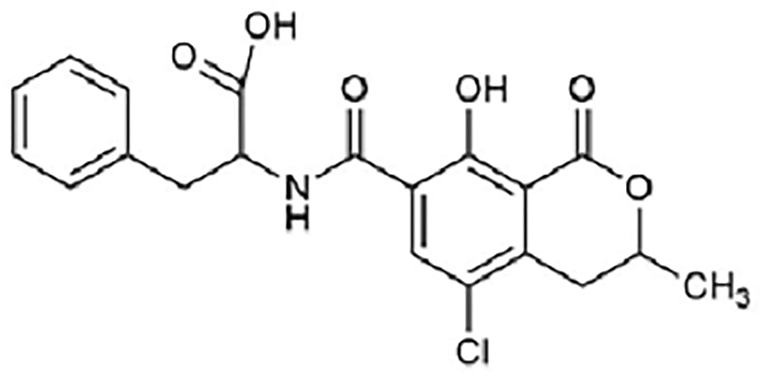
Ochratoxin A (Figure 3) is a powerful mycotoxin, responsible for chronic toxicities such as nephrotoxicity, hepatotoxicity, teratogenicity, and immunotoxicity in humans. [18] There are several in vivo and in vitro studies published regarding nephrotoxicity and hepatotoxicity of OchA, due to diverse metabolites of OchA, but the exact mechanism of toxicity of this mycotoxin is still unclear. [19, 20]
|
As such, the aim of this study was to investigate whether the cannabis extract obtained from cannabis flowers that contain the maximum allowed level of mycotoxins affects human safety and health. There are many recently published manuscripts related to quantification techniques for the determination of mycotoxins in cannabis flower and cannabis extracts; however, their methods are inapplicable for the equipment we use. [21, 22] For that reason, a novel method of liquid chromatography with tandem mass spectrometry (LC-MS/MS) was developed and validated for the determination of aflatoxins and OchA in cannabis extracts to demonstrate that this analytical method is suitable for the intended experimental design, thus, achieving the set goal.
Materials and methods
Chemicals and reagents
Liquid standards of AfB1 Cat.No.TSL-104-10, aflatoxin B2 (AfB2) Cat.No.TSL-105-10, aflatoxin G1 (AfG1) Cat.No.TSL-106-10, aflatoxin G2 (AfG2) Cat.No.TSL-107-10, and OchA Cat.No.TSL-504-5 were supplied by R-Biopharm (Germany). Other chemicals and reagents used in this study were LC/MS-grade and provided by Fisher Chemicals (U.K.).
Immunoaffinity columns (IAC) were obtained from R-Biopharm (Germany), Cat. No. RBRP112B.
Apparatus
Liquid chromatography was performed on an LC-MS/MS system (LC - 30AD series) equipped with a binary pump, vacuum degasser, standard autosampler, column compartment, and MS/MS detector (8045 series) from Shimadzu (Japan).
Chromatographic conditions
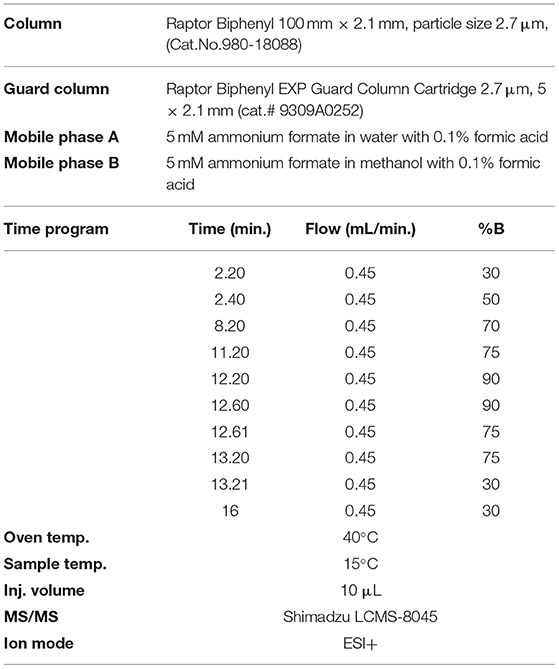
The separation was performed using a gradient method and reversed-phase Raptor Biphenyl LC column (100 x 2.1 mm, particle size 2.7 μm, Cat.No.980-18088) that provide the best separation characteristics, coupled with Raptor Biphenyl EXP guard column cartridge (5 x 2.1 mm, particle size 2.7 μm, cat.No.9309A0252) from Restek (USA). The choice of solvent for the preparation of standard solutions and extraction of mycotoxins (aflatoxins and OchA) from cannabis floss and extracts was made to eliminate the isobaric interferences resulting in spiked samples. It was determined that an extraction solvent of 50:50 = water:methanol with 0.1% formic acid results in samples (extracts) with removed isobaric interferences.
The mobile phase contains a mixture of 5 mM ammonium formate in water with 0.1% formic acid and 5 mM ammonium formate in methanol with 0.1% formic acid, and a flow rate of 0.45 ml/min gave the best results. Analytical conditions for the gradient method are given in Table 1. The injection volume of samples was 10 μl.
|
Water:methanol = 50:50 (V/V) with 0.1% formic acid was used for the preparation of standard solutions and extraction of mycotoxins (aflatoxins and OchA) from cannabis flowers and extracts.
Chromatographic data were analyzed using LabSolution software from Shimadzu (Japan), following the requirements for chromatographic analysis.
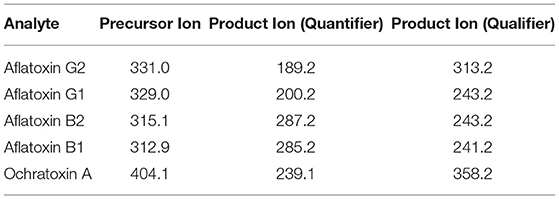
Analyte transitions are given in Table 2.
|
Sample preparation
About 2 g of sample (ground flowers or extract) was weighed out in a 50 ml centrifuge tube and then a 15 ml mixture of methanol:water (80:20) was added to the tube. The mixture was shaken vigorously for 60 minutes using the rotation shaker (70 rpm/min) and then centrifuged at 4,000 rpm for 15 minutes. About 6 ml of the supernatant was transferred to a new tube containing 20 ml of 2% Tween wash buffer in phosphate-buffered saline (PBS) and mixed well. A total of 26 ml of the diluted extract was passed through the IAC AOZON at a rate of 1 drop per second until air came through the column (it is important that the flow rate does not exceed 1 drop per second). The column was washed with three portions of 15 ml water (MS grade). Finally, the column was washed with 1 ml of methanol. That 1 ml is then diluted with 1 ml 0.2% formic acid. The sample solutions before injection in the system were filtered using a membrane filter (0.2 μm pore size).
Standard solutions and calibration curves
Working calibration solutions containing AflB1, AflB2, AflG1, and AflG2 were prepared from 0.1–5 μg/L in 50:50 water:methanol with 0.1% formic acid, and OchA was prepared from 1–50μg/L in 50:50 water:methanol with 0.1% formic acid.
Due to the influence of the matrix on the result (difference in the slope of the curve between the standard prepared in methanol water 50:50 with 0.1% formic acid and the standard prepared in the matrix), the calibration curve was made in a matrix.
Validation of the method
The proposed method was validated according to the guidelines set by the International Conference of Harmonization for validation of analytical methods and Directive 96/23/EC considering the performance of analytical methods and the interpretation of results. [23, 24]
Recovery studies
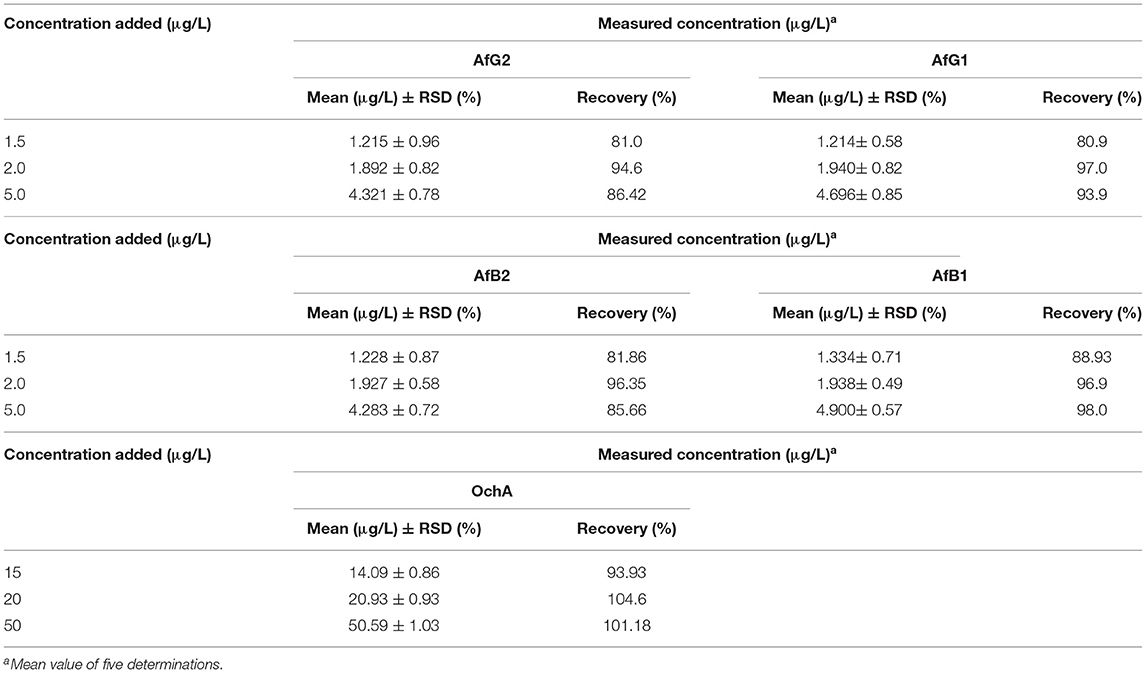
To establish the accuracy of the proposed method, recovery experiments were carried out by adding the known amounts of the combined standard solution of AflB1, AflB2, AflG1, AflG2, and OchA to the decarboxylated oil.
Experimental design
At the beginning of the experiment, the concentration of AfB1, AfB2, AfG1, AfG2, and Och A in dry cannabis flowers (we use a variety of Herijuana) was determined. Then, AfB1, AfB2, AfG1, AfG2 (2 μg/kg), and Och A (20 μg/kg) were added to 250 g dried cannabis flowers, which were divided into three equal portions 2 hours after adding mycotoxins. The maceration was performed with 96% ethanol as a solvent, in a cold chamber (refrigerator at −20°C). The duration of the maceration was 30 minutes in total. Each portion of cannabis flowers was macerated in approximately 415 ml of 96% ethanol for 10 minutes separately (in total, 1.25 L of 96% ethanol was used for maceration). Stirring by stainless steel spoon was done on portion every 2 minutes. After maceration was completed, the macerated material (cannabis flowers) was manually squeezed with a stainless-steel strainer. The resulting macerate was filtered and collected in a pre-measured 1L beaker. The ethanol was evaporated on a hot plate. After evaporation of the ethanol, the obtained crude oil was decarboxylated by heating until the temperature of the crude extract reached 125–130°C. The experiment was performed three times (Batch No. RS0221/1, Batch No. RS0221/2, Batch No. RS0221/3).
Results
Validation of the method
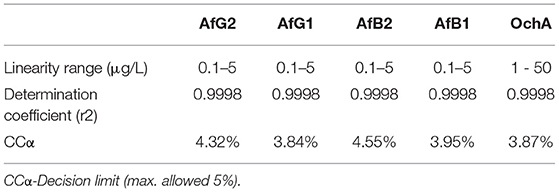
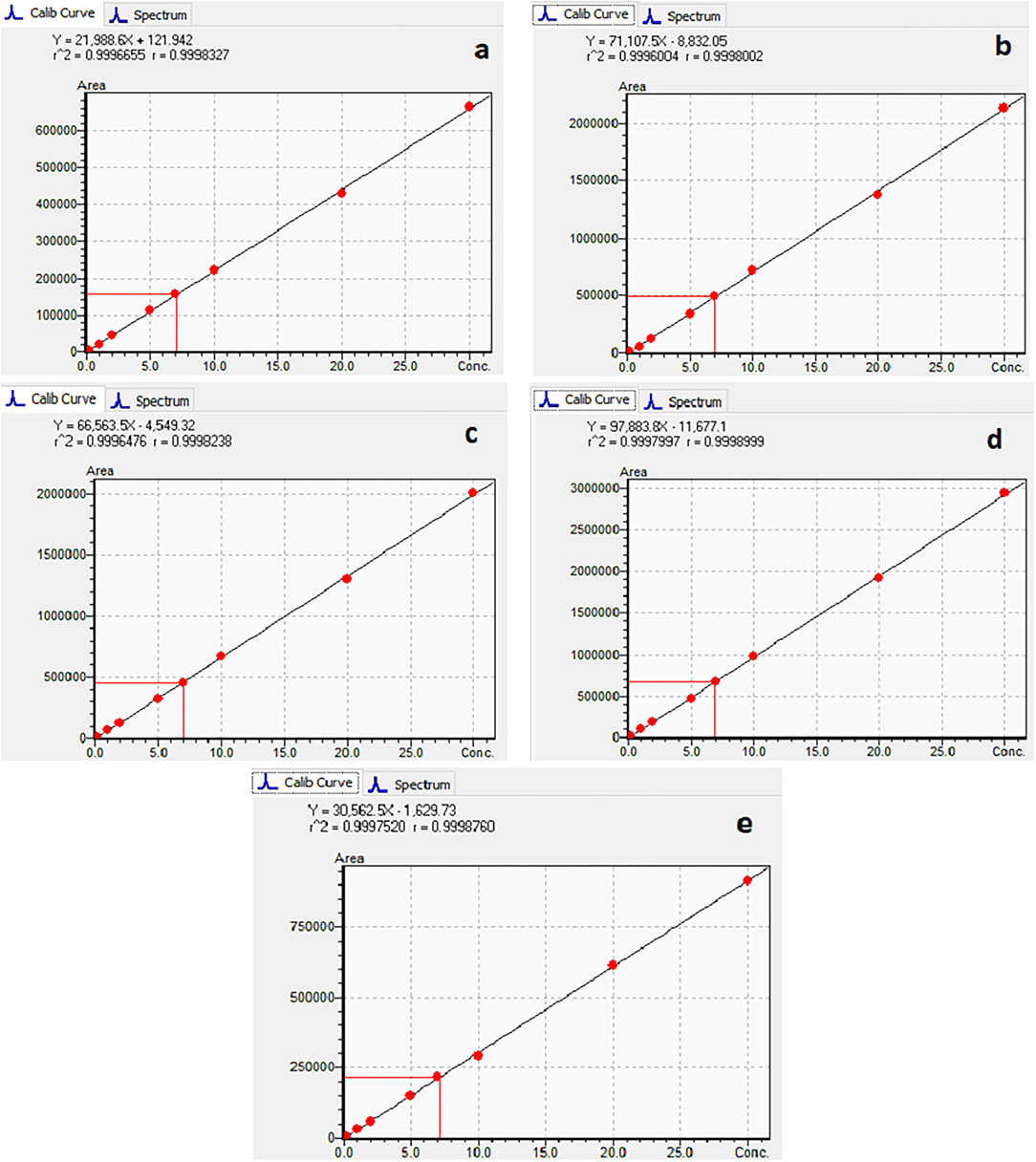
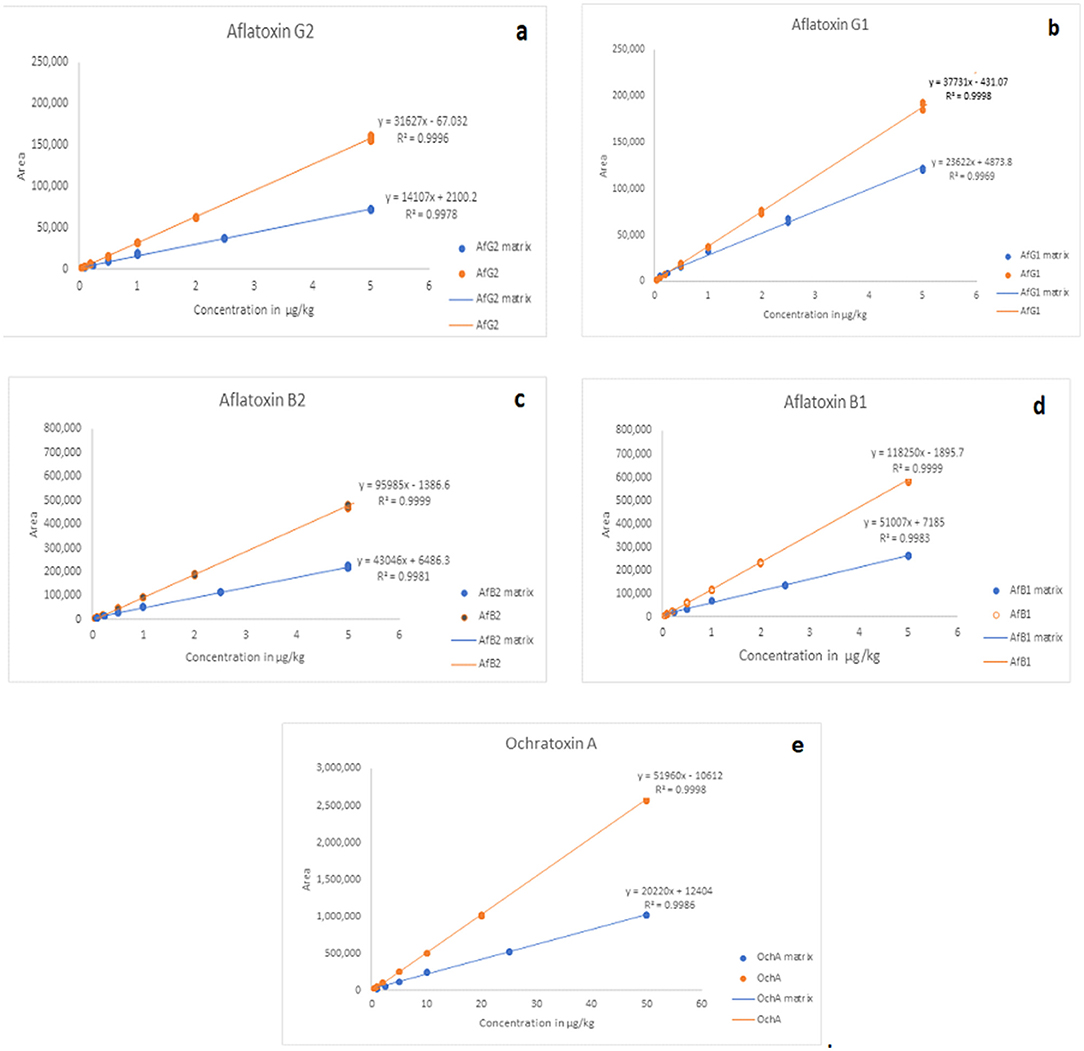
The calibration characteristics and validation parameters of the proposed method are shown in Table 3 and Figure 4. Linearity of response was calculated as a ratio of peak areas of AflB1, AflB2, AflG1, AflG2, and OchA vs. concentration in the spiked samples in the concentration range of 0.1–5 μg/L in 50:50 water:methanol with 0.1% formic acid for AflB1, AflB2, AflG1, and AflG2, and OchA from 1–50 μg/L in 50:50 water:methanol with 0.1% formic acid. The correlation coefficient was >0.999 for all mycotoxins.
|
|
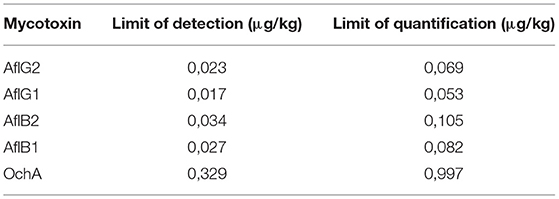
The limit of detection (DL)/limit of quantification (QL) was determined based on the formulas: DL = 3,3 x σ / S and QL = 10 x σ / S, where σ is the SD of the response and S is the slope of the calibration curve. The DL/QL for each separate mycotoxin is shown in Table 4.
|
Figure 4 shows the calibration curve of AfG2 (4-a), the calibration curve of AfG1 (4-b), the calibration curve of AfB2 (4-c), the calibration curve of AfB1 (4-d), and the calibration curve of OchA (4-e).
The results of precision, accuracy (recovery), and reproducibility (assay) of the method are shown in Table 5. They demonstrate good precision determined as the relative standard deviation (%RSD) accuracy, and reproducibility. RSD is the absolute value of the coefficient of variation and indicates whether the “regular” SD is a small or large quantity when compared to the mean for the data set.
|
The matrix effect was evaluated using the post-extraction spike method, by adding standard solutions of mycotoxins into the matrix after extraction. Calibration curves of standard solutions of mycotoxins and spiked standard solutions of mycotoxins in the matrix were evaluated. It was shown that co-eluting compounds from the matrix affect the ionization efficiency and reproducibility in the ionization source (Figure 5). Therefore, all analyses were evaluated using matrix calibration curves.
|
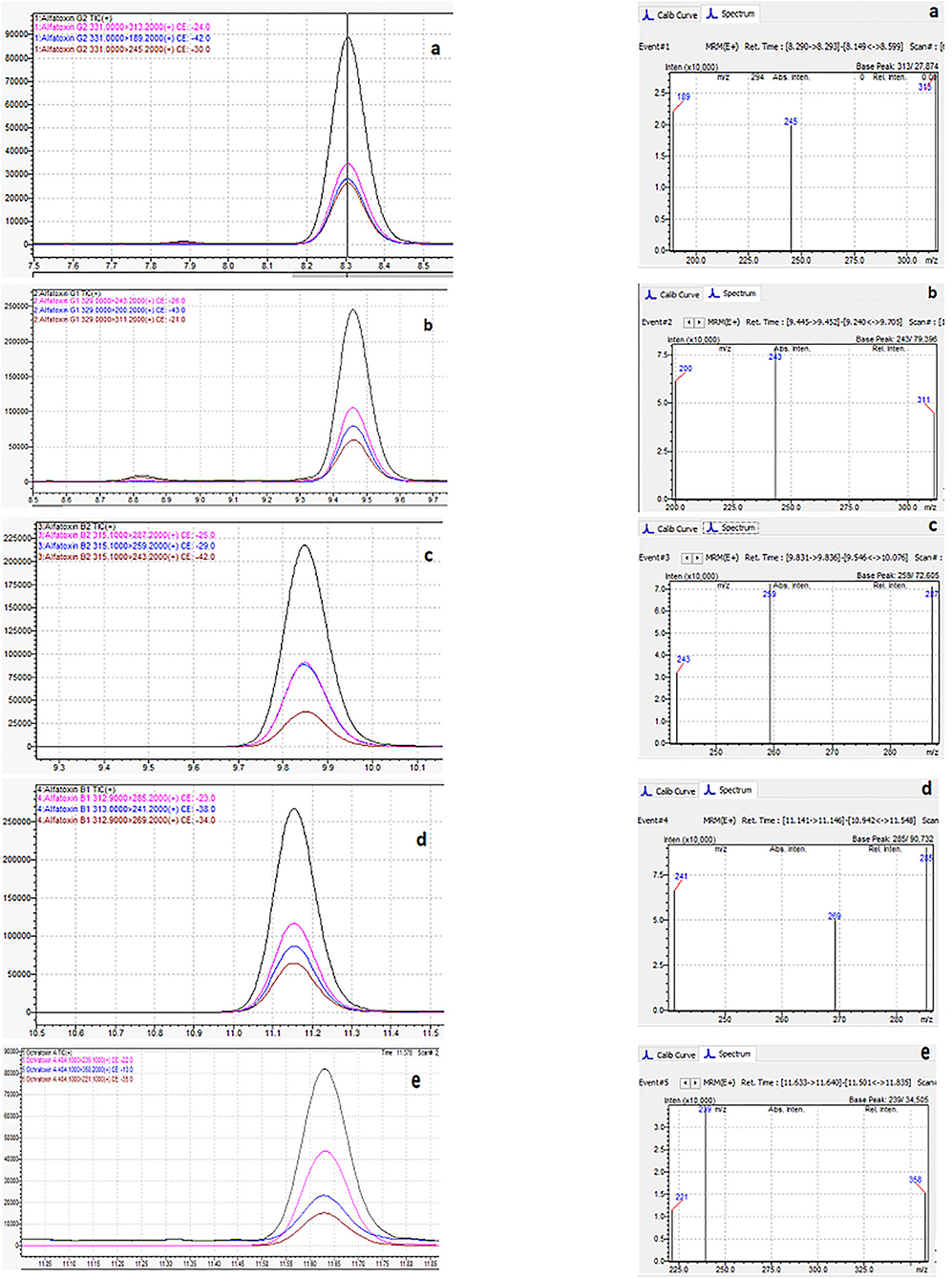
Figure 6 shows the typical chromatogram and analyte transitions of AfG2 (5-a), the typical chromatogram and analyte transitions of AfG1 (5-b), the typical chromatogram and analyte transitions of AfB2 (5-c), the typical chromatogram and analyte transitions of AfB1 (5-d), and the typical chromatogram and analyte transitions of OchA (5-e).
|
Calculation of AflB1, AflB2, AflG1, AflG2, and OchA in experimental design
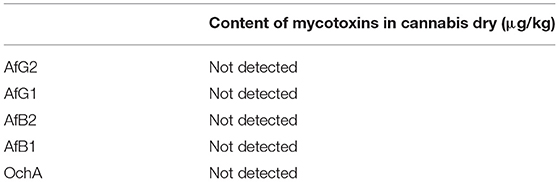
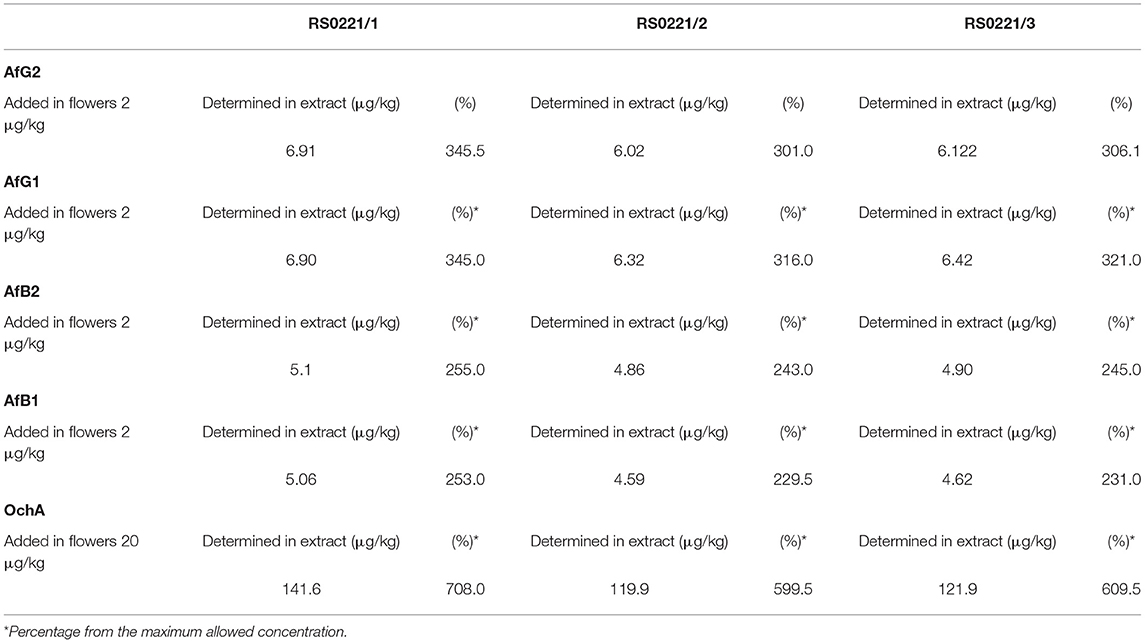
The concentration of aflatoxins and OchA were determined using the same LC-MS/MS analytical method in the starting material (dry flower) before spike and in obtained decarboxylated extract after extraction with ethanol 96%. The content of AflB1, AflB2, AflG1, AflG2, and OchA in micrograms per kilogram of cannabis flower variety Herijuana (Table 6), and cannabis extracts (Table 7) obtained from cannabis flowers spiked with maximum residual level (MRL) allowed for aflatoxins (2 μg/kg) and (20 μg/kg) for OchA was calculated separately for each mycotoxin.
|
|
The results of the assay in the not-spiked dry flowers (Table 6) show that there are no significant quantities of mycotoxins that could influence the validity of the experiment.
From the percent presented in Table 7, we can conclude that after evaporation of the solvent, aflatoxins and OchA remain in the final extract in amounts much higher than those in which the same are added.
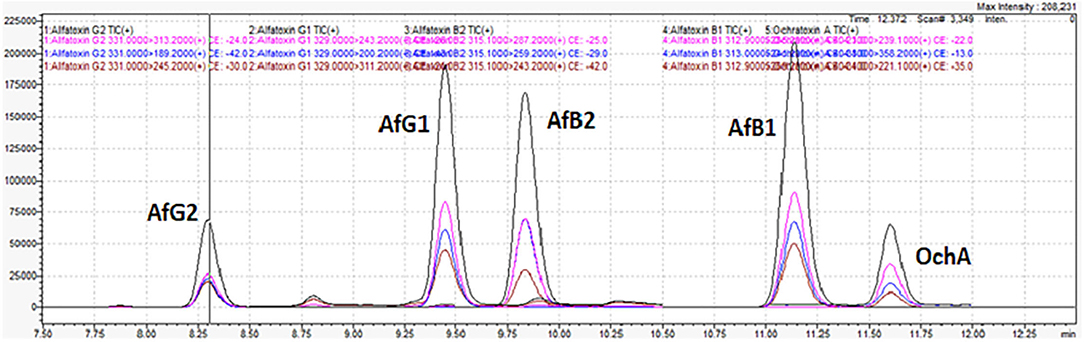
Figure 7 shows the chromatogram of cannabis extract obtained from cannabis flowers spiked with MRL for aflatoxins (2 μg/kg) and OchA (20 μg/kg).
|
Discussion
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added.