Difference between revisions of "Journal:Does cannabis extract obtained from cannabis flowers with maximum allowed residual level of aflatoxins and ochratoxin A have an impact on human safety and health?"
Shawndouglas (talk | contribs) (Created stub; saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 6: | Line 6: | ||
|title_full = Does cannabis extract obtained from cannabis flowers with maximum allowed residual level of aflatoxins and ochratoxin A have an impact on human safety and health? | |title_full = Does cannabis extract obtained from cannabis flowers with maximum allowed residual level of aflatoxins and ochratoxin A have an impact on human safety and health? | ||
|journal = ''Frontiers in Medicine'' | |journal = ''Frontiers in Medicine'' | ||
|authors = | |authors = Serafimovska, Tijana; Stefanovski, Sasho; Erler, Joachim; Keskovski, Zlatko; Stefkov, Gjoshe; Mitevska, Marija; Serafimovska, Marija D.; Balkanov, Trajan; Ribarska, Jasmina T. | ||
|affiliations = Ss. Cyril and Methodius University of Skopje, NYSK Holdings, Diapharm GmbH & Co. KG, Goce Delchev University | |affiliations = Ss. Cyril and Methodius University of Skopje, NYSK Holdings, Diapharm GmbH & Co. KG, Goce Delchev University | ||
|contact = Email: serafimovskatijana at gmail dot com | |contact = Email: serafimovskatijana at gmail dot com | ||
| Line 26: | Line 26: | ||
}} | }} | ||
==Abstract== | ==Abstract== | ||
'''Aim''': The aim of this study was to investigate whether the [[Cannabis concentrate|cannabis extract]] obtained from [[cannabis]] flowers that contain the maximum allowed level of [[mycotoxin]]s affects human safety and health. For that purpose, a novel method of [[Chromatography#Liquid chromatography|liquid chromatography]] with [[tandem mass spectrometry]] (LC-MS/MS) was developed and validated for the determination of [[aflatoxin]]s and [[Ochratoxin|ochratoxin A]] (OchA) in cannabis extracts to demonstrate that this analytical method is suitable for the intended experimental design. | '''Aim''': The aim of this study was to investigate whether the [[Cannabis concentrate|cannabis extract]] obtained from [[cannabis]] [[Inflorescence|flowers]] that contain the maximum allowed level of [[mycotoxin]]s affects human safety and health. For that purpose, a novel method of [[Chromatography#Liquid chromatography|liquid chromatography]] with [[tandem mass spectrometry]] (LC-MS/MS) was developed and validated for the determination of [[aflatoxin]]s and [[Ochratoxin|ochratoxin A]] (OchA) in cannabis extracts to demonstrate that this analytical method is suitable for the intended experimental design. | ||
'''Methods''': Experimental design was done by adding maximum allowed concentration of aflatoxins (B1, B2, G1, G2) and OchA according to the European Pharmacopeia related to cannabis flowers. The concentration of aflatoxins and OchA was determined using the same LC-MS/MS analytical method on the initial plant material (dry flower) before preparing the spiked [[Sample (material)|sample]] and after obtaining [[Decarboxylation|decarboxylated]] extract with ethanol 96%. | '''Methods''': Experimental design was done by adding maximum allowed concentration of aflatoxins (B1, B2, G1, G2) and OchA according to the ''European Pharmacopeia'' related to cannabis flowers. The concentration of aflatoxins and OchA was determined using the same LC-MS/MS analytical method on the initial plant material (dry flower) before preparing the spiked [[Sample (material)|sample]] and after obtaining [[Decarboxylation|decarboxylated]] extract with ethanol 96%. | ||
'''Results''': The results obtained indicate that aflatoxins and OchA, primarily added to the cannabis dried flowers, were also determined into the obtained final extract in amounts much higher (m/m) than in the initial plant material. | '''Results''': The results obtained indicate that aflatoxins and OchA, primarily added to the cannabis dried flowers, were also determined into the obtained final extract in amounts much higher (m/m) than in the initial plant material. | ||
| Line 37: | Line 37: | ||
==Introduction== | ==Introduction== | ||
In traditional medicine, medicinal products based on [[Cannabis sativa]] L. (Cannabaceae) have been used for thousands of years in the treatment of various diseases. [1] Although, there is a lack of evidence-based medical information that can prove the potential benefit of the therapy with [[Cannabis (drug)|medicinal cannabis]] preparations, recently, an increasing number of pharmacists have issued cannabis preparations to individual patients prescribed by their physicians. [2] | |||
To obtain [[cannabis]]-based preparations, standardized concentrated [[cannabinoid]] [[Cannabis concentrate|extracts]], produced by a suitable extraction process of cannabinoids from cannabis [[Inflorescence|flowers]], are used. [2] The safety of the flowers as a starting material, in this case, is of the utmost significance for human safety and health. Since there is no monograph in the ''European Pharmacopeia'' for [[Quality control|quality testing]] of cannabis flowers, currently a revised monograph for cannabis flower (cannabis floss), published by the German Federal Institute for Drugs and Medical Devices (BfArM) in the ''German Pharmacopoeia'' [3], instructs the obligatory procedure for quality testing of cannabis flowers in the European Union. [4] However, a variety of herbal monographs are listed under the general monographs in the ''European Pharmacopeia'': herbal drugs, herbal drug extracts, and herbal drug preparations. These general monographs are created to cover products and quality parameters, which are not mentioned in the individual monographs. Therefore, it is necessary to apply the individual monograph always in combination with these quality requirements. [5] | |||
According to the European Medicines Agency's "Guideline on specifications: test procedures and acceptance criteria for herbal substances, herbal preparations and herbal medicinal products" [6], [[mycotoxin]]s (i.e., [[aflatoxin]]s and [[Ochratoxin|ochratoxin A]] [OchA]) are considered impurities ([[Contamination|contaminants]]) that can occur in the final extracts from their initial plant materials (herbal drugs). In reference to this, the ''European Pharmacopeia'' gives the maximum allowed limits of these impurities (aflatoxins, as per ''European Pharmacopeia'' 2.8.18, and OchA, as per ''European Pharmacopeia'' 2.8.22) in herbal drugs. | |||
===Impact of mycotoxins on human safety and health=== | |||
Mycotoxins like aflatoxins and OchA are secondary toxic [[Metabolomics|metabolites]], obtained primarily from fungal species (''[[Penicillium]]'' and ''[[Aspergillus]]''). Fungi and their metabolites contaminate the raw materials usually used in the preparation of products for human use. [7] The presence of these contaminants in products for human use can cause various acute and chronic effects on human health and safety. [8] | |||
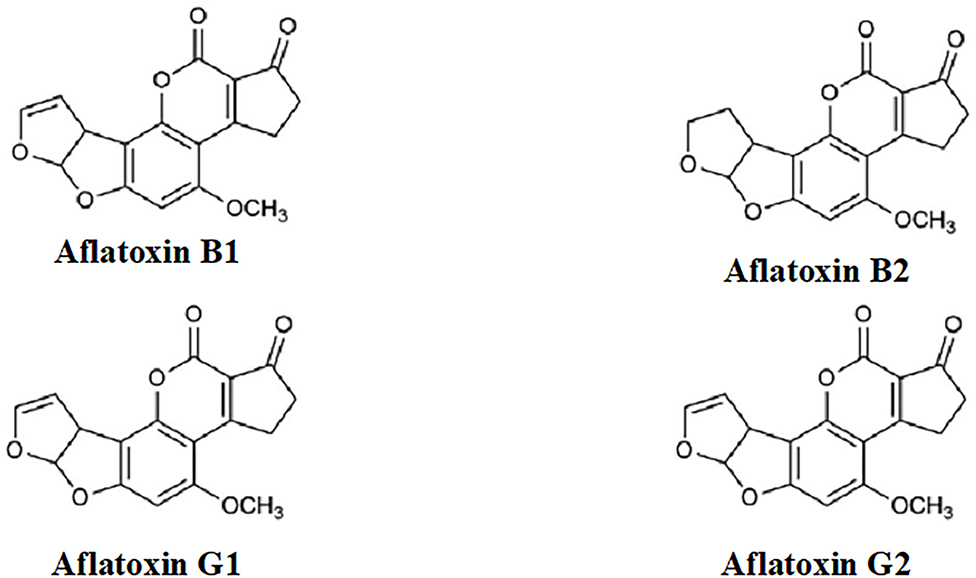
Aflatoxins (Figure 1) are extremely toxic secondary metabolites. According to their chemical structures, they are generally categorized into two groups: the difurocoumarocyclopentenone group (aflatoxin B1 and B2) and the difurocoumarolactone group (aflatoxin G1 and G2). The most toxic, carcinogenic, and mutagenic among all the aflatoxins is aflatoxin B1 (AfB1). Humans are mostly infected by direct ingestion (consuming) of infected herbal drugs, food, or herbal preparations. [9] | |||
[[File:Fig1 Serafimovska FrontMed2021 8.jpg|600px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="600px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Fig. 1''' Structures of aflatoxins</blockquote> | |||
|- | |||
|} | |||
|} | |||
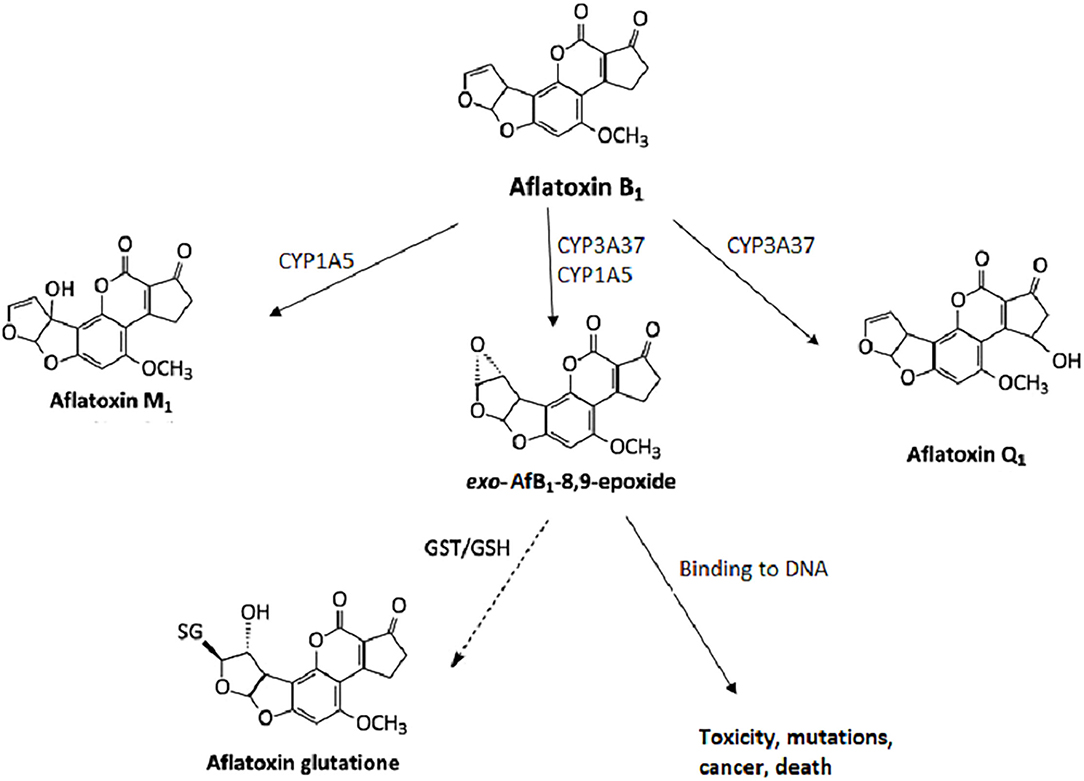
Mycotoxins are metabolized in the liver, and microorganisms in the digestive tract. [10, 11] AfB1 itself is not carcinogenic, but in the human body, it is metabolized into carcinogenic metabolites. All enzymes necessary for the bioactivation of AfB1 are present in the nuclear envelope of the hepatocytes. The mono-oxygenase system in microsomes can transform the AfB1 into aflatoxin M1 (AfM1), aflatoxin Q1 (AfQ1), and exo-AfB1-8,9-epoxide (Figure 2), which can bind to deoxyribonucleic acid (DNA) and causes cytotoxicity, DNA damage, chromosomal anomalies, gene mutation, and cell transformation by attacking the nucleophilic hetero-atoms such as oxygen and nitrogen in the organic bases of nucleic acids and forming a strong covalent bond to the DNA. [9] | |||
[[File:Fig2 Serafimovska FrontMed2021 8.jpg|800px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="800px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Fig. 2''' Metabolism of aflatoxin B1 in hepatocytes</blockquote> | |||
|- | |||
|} | |||
|} | |||
Specific effects of aflatoxins on human safety and health can be classified into two groups: chronic toxicity and acute toxicity. | |||
Carcinogenicity, hepatotoxicity, nephrotoxicity, and endocrine disorders have been related to chronic exposure to low levels of mycotoxins. [12, 13] In some cases, allergic reactions, immune diseases, reproductive deficiencies, fetal alterations, and death have also been related to chronic exposure to mycotoxins. [14] The health risks associated with mycotoxin exposure arise from their toxicity and depend on the type of toxin, its metabolism, the immune system, and the health status of the exposed individual. [15] Due to the carcinogenic risk associated with the mycotoxins, the International Agency for Research on Cancer (IARC) has evaluated and classified mycotoxins as carcinogenic to humans (Group 1), possibly carcinogenic to humans (Group 2B), or not classifiable as to its carcinogenicity to humans (Group 3), based on sufficient experimental data. [16] The main target organ for toxicity, mutagenicity, and carcinogenicity is the liver. [17] | |||
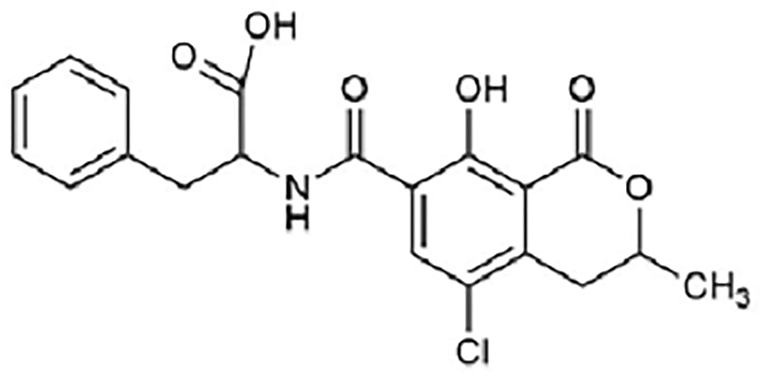
Ochratoxin A (Figure 3) is a powerful mycotoxin, responsible for chronic toxicities such as nephrotoxicity, hepatotoxicity, teratogenicity, and immunotoxicity in humans. [18] There are several ''in vivo'' and ''in vitro'' studies published regarding nephrotoxicity and hepatotoxicity of OchA, due to diverse metabolites of OchA, but the exact mechanism of toxicity of this mycotoxin is still unclear. [19, 20] | |||
[[File:Fig3 Serafimovska FrontMed2021 8.jpg|600px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="600px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Fig. 3''' Structures of ochratoxin A</blockquote> | |||
|- | |||
|} | |||
|} | |||
As such, the aim of this study was to investigate whether the cannabis extract obtained from cannabis flowers that contain the maximum allowed level of mycotoxins affects human safety and health. There are many recently published manuscripts related to quantification techniques for the determination of mycotoxins in cannabis flower and cannabis extracts; however, their methods are inapplicable for the equipment we use. [21, 22] For that reason, a novel method of [[Chromatography#Liquid chromatography|liquid chromatography]] with [[tandem mass spectrometry]] (LC-MS/MS) was developed and validated for the determination of aflatoxins and OchA in cannabis extracts to demonstrate that this analytical method is suitable for the intended experimental design, thus, achieving the set goal. | |||
==Materials and methods== | |||
| Line 49: | Line 106: | ||
[[Category:CannaQAwiki journal articles (added in 2022)]] | [[Category:CannaQAwiki journal articles (added in 2022)]] | ||
[[Category:CannaQAwiki journal articles (all)]] | [[Category:CannaQAwiki journal articles (all)]] | ||
[[Category:CannaQAwiki journal articles on cannabis | [[Category:CannaQAwiki journal articles on cannabis contaminants]] | ||
[[Category:CannaQAwiki journal articles on cannabis research]] | [[Category:CannaQAwiki journal articles on cannabis research]] | ||
[[Category:CannaQAwiki journal articles on cannabis testing]] | [[Category:CannaQAwiki journal articles on cannabis testing]] | ||
Revision as of 19:33, 19 February 2022
| Full article title | Does cannabis extract obtained from cannabis flowers with maximum allowed residual level of aflatoxins and ochratoxin A have an impact on human safety and health? |
|---|---|
| Journal | Frontiers in Medicine |
| Author(s) | Serafimovska, Tijana; Stefanovski, Sasho; Erler, Joachim; Keskovski, Zlatko; Stefkov, Gjoshe; Mitevska, Marija; Serafimovska, Marija D.; Balkanov, Trajan; Ribarska, Jasmina T. |
| Author affiliation(s) | Ss. Cyril and Methodius University of Skopje, NYSK Holdings, Diapharm GmbH & Co. KG, Goce Delchev University |
| Primary contact | Email: serafimovskatijana at gmail dot com |
| Editors | Bolcato, Matteo |
| Year published | 2021 |
| Volume and issue | 8 |
| Article # | 759856 |
| DOI | 10.3389/fmed.2021.759856 |
| ISSN | 2296-858X |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.frontiersin.org/articles/10.3389/fmed.2021.759856/full |
| Download | https://www.frontiersin.org/articles/10.3389/fmed.2021.759856/pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Aim: The aim of this study was to investigate whether the cannabis extract obtained from cannabis flowers that contain the maximum allowed level of mycotoxins affects human safety and health. For that purpose, a novel method of liquid chromatography with tandem mass spectrometry (LC-MS/MS) was developed and validated for the determination of aflatoxins and ochratoxin A (OchA) in cannabis extracts to demonstrate that this analytical method is suitable for the intended experimental design.
Methods: Experimental design was done by adding maximum allowed concentration of aflatoxins (B1, B2, G1, G2) and OchA according to the European Pharmacopeia related to cannabis flowers. The concentration of aflatoxins and OchA was determined using the same LC-MS/MS analytical method on the initial plant material (dry flower) before preparing the spiked sample and after obtaining decarboxylated extract with ethanol 96%.
Results: The results obtained indicate that aflatoxins and OchA, primarily added to the cannabis dried flowers, were also determined into the obtained final extract in amounts much higher (m/m) than in the initial plant material.
Conclusion: With this experiment, we have shown that mycotoxins, especially aflatoxins, which are extremely toxic secondary metabolites, can reach critical values in cannabis extracts obtained from dry cannabis flowers with the maximum allowed quantity of mycotoxins. This can pose a great risk to consumers and their health, especially to those with compromised immune systems.
Keywords: mycotoxins, aflatoxins, ochratoxin A, determination liquid chromatography with tandem mass spectrometry (LC/MS/MS), cannabis extracts
Introduction
In traditional medicine, medicinal products based on Cannabis sativa L. (Cannabaceae) have been used for thousands of years in the treatment of various diseases. [1] Although, there is a lack of evidence-based medical information that can prove the potential benefit of the therapy with medicinal cannabis preparations, recently, an increasing number of pharmacists have issued cannabis preparations to individual patients prescribed by their physicians. [2]
To obtain cannabis-based preparations, standardized concentrated cannabinoid extracts, produced by a suitable extraction process of cannabinoids from cannabis flowers, are used. [2] The safety of the flowers as a starting material, in this case, is of the utmost significance for human safety and health. Since there is no monograph in the European Pharmacopeia for quality testing of cannabis flowers, currently a revised monograph for cannabis flower (cannabis floss), published by the German Federal Institute for Drugs and Medical Devices (BfArM) in the German Pharmacopoeia [3], instructs the obligatory procedure for quality testing of cannabis flowers in the European Union. [4] However, a variety of herbal monographs are listed under the general monographs in the European Pharmacopeia: herbal drugs, herbal drug extracts, and herbal drug preparations. These general monographs are created to cover products and quality parameters, which are not mentioned in the individual monographs. Therefore, it is necessary to apply the individual monograph always in combination with these quality requirements. [5]
According to the European Medicines Agency's "Guideline on specifications: test procedures and acceptance criteria for herbal substances, herbal preparations and herbal medicinal products" [6], mycotoxins (i.e., aflatoxins and ochratoxin A [OchA]) are considered impurities (contaminants) that can occur in the final extracts from their initial plant materials (herbal drugs). In reference to this, the European Pharmacopeia gives the maximum allowed limits of these impurities (aflatoxins, as per European Pharmacopeia 2.8.18, and OchA, as per European Pharmacopeia 2.8.22) in herbal drugs.
Impact of mycotoxins on human safety and health
Mycotoxins like aflatoxins and OchA are secondary toxic metabolites, obtained primarily from fungal species (Penicillium and Aspergillus). Fungi and their metabolites contaminate the raw materials usually used in the preparation of products for human use. [7] The presence of these contaminants in products for human use can cause various acute and chronic effects on human health and safety. [8]
Aflatoxins (Figure 1) are extremely toxic secondary metabolites. According to their chemical structures, they are generally categorized into two groups: the difurocoumarocyclopentenone group (aflatoxin B1 and B2) and the difurocoumarolactone group (aflatoxin G1 and G2). The most toxic, carcinogenic, and mutagenic among all the aflatoxins is aflatoxin B1 (AfB1). Humans are mostly infected by direct ingestion (consuming) of infected herbal drugs, food, or herbal preparations. [9]
|
Mycotoxins are metabolized in the liver, and microorganisms in the digestive tract. [10, 11] AfB1 itself is not carcinogenic, but in the human body, it is metabolized into carcinogenic metabolites. All enzymes necessary for the bioactivation of AfB1 are present in the nuclear envelope of the hepatocytes. The mono-oxygenase system in microsomes can transform the AfB1 into aflatoxin M1 (AfM1), aflatoxin Q1 (AfQ1), and exo-AfB1-8,9-epoxide (Figure 2), which can bind to deoxyribonucleic acid (DNA) and causes cytotoxicity, DNA damage, chromosomal anomalies, gene mutation, and cell transformation by attacking the nucleophilic hetero-atoms such as oxygen and nitrogen in the organic bases of nucleic acids and forming a strong covalent bond to the DNA. [9]
|
Specific effects of aflatoxins on human safety and health can be classified into two groups: chronic toxicity and acute toxicity.
Carcinogenicity, hepatotoxicity, nephrotoxicity, and endocrine disorders have been related to chronic exposure to low levels of mycotoxins. [12, 13] In some cases, allergic reactions, immune diseases, reproductive deficiencies, fetal alterations, and death have also been related to chronic exposure to mycotoxins. [14] The health risks associated with mycotoxin exposure arise from their toxicity and depend on the type of toxin, its metabolism, the immune system, and the health status of the exposed individual. [15] Due to the carcinogenic risk associated with the mycotoxins, the International Agency for Research on Cancer (IARC) has evaluated and classified mycotoxins as carcinogenic to humans (Group 1), possibly carcinogenic to humans (Group 2B), or not classifiable as to its carcinogenicity to humans (Group 3), based on sufficient experimental data. [16] The main target organ for toxicity, mutagenicity, and carcinogenicity is the liver. [17]
Ochratoxin A (Figure 3) is a powerful mycotoxin, responsible for chronic toxicities such as nephrotoxicity, hepatotoxicity, teratogenicity, and immunotoxicity in humans. [18] There are several in vivo and in vitro studies published regarding nephrotoxicity and hepatotoxicity of OchA, due to diverse metabolites of OchA, but the exact mechanism of toxicity of this mycotoxin is still unclear. [19, 20]
|
As such, the aim of this study was to investigate whether the cannabis extract obtained from cannabis flowers that contain the maximum allowed level of mycotoxins affects human safety and health. There are many recently published manuscripts related to quantification techniques for the determination of mycotoxins in cannabis flower and cannabis extracts; however, their methods are inapplicable for the equipment we use. [21, 22] For that reason, a novel method of liquid chromatography with tandem mass spectrometry (LC-MS/MS) was developed and validated for the determination of aflatoxins and OchA in cannabis extracts to demonstrate that this analytical method is suitable for the intended experimental design, thus, achieving the set goal.
Materials and methods
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added.