Difference between revisions of "Journal:Chemometric analysis of cannabinoids: Chemotaxonomy and domestication syndrome"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 41: | Line 41: | ||
===Targeted metabolomics of cannabinoids=== | ===Targeted metabolomics of cannabinoids=== | ||
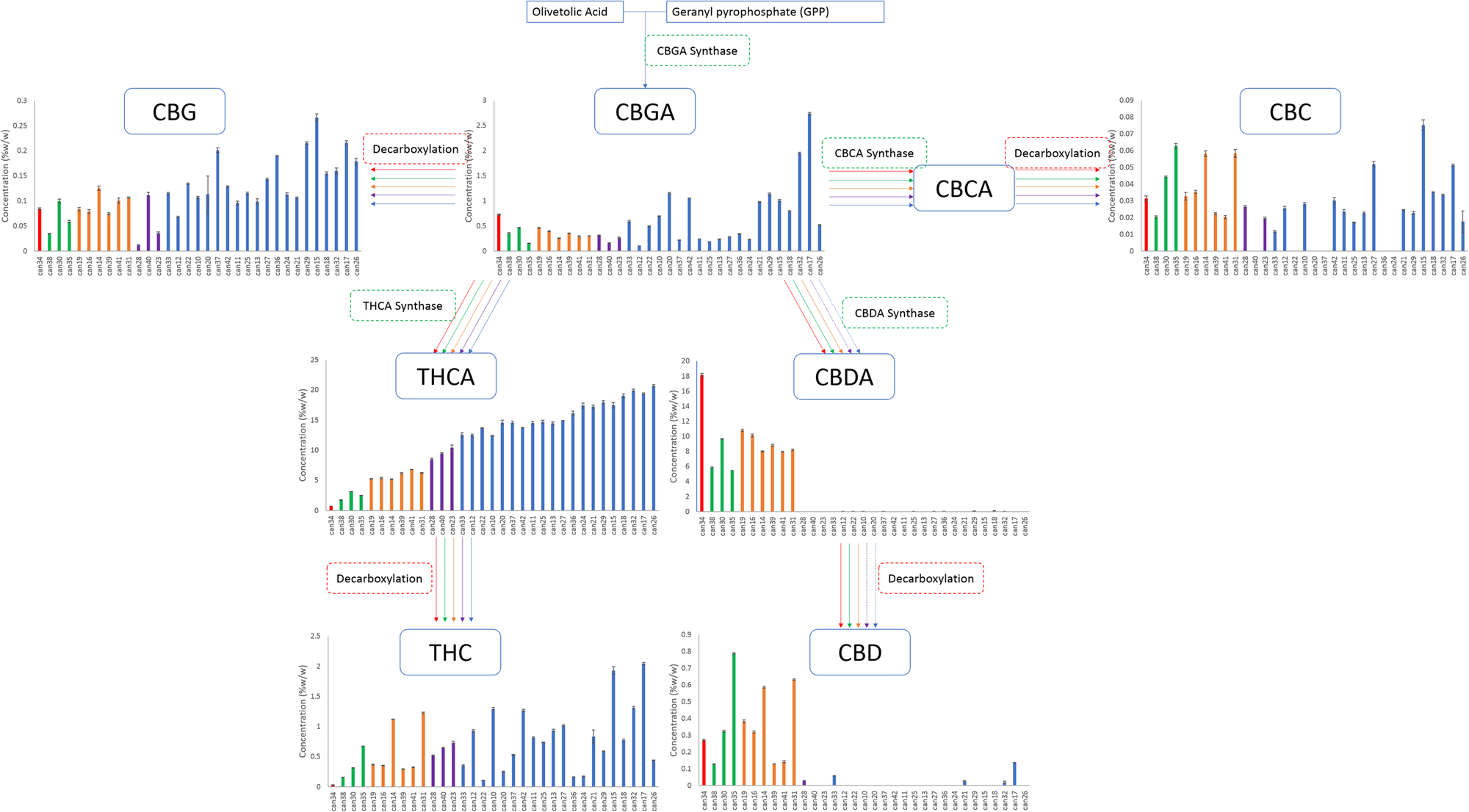
Two cannabinoids for which standards were obtained, [[cannabidivarin]] (CBDV) and [[cannabicyclol]] (CBL), were not detected in any strain. The 11 remaining cannabinoids with available chemical reference standards were identified and quantified. THCA content ranged from 0.76 to 20.71% w/w, with almost a linear increase in content from the lowest to highest strain with an r2 of 0.97, while CBDA content ranged from <MDL to 18.11% w/w, with the highest CBDA strains having the lowest THCA contents (Fig. 1). In THC-abundant strains, the CBDA levels were less than 0.15%, while in CBD abundant strains the content was greater than 5%. THC, the decarboxylated form of THCA, was present in strains from <LOQ up to 2% by weight in some strains, while CBD contents ranged from <MDL to 0.8%. CBD was most prevalent in high-CBDA strains. In addition, seven cannabinoids present at lower levels were quantified using individual calibration standards: [[tetrahydrocannabivarin]] (THCV), [[cannabigerol]] (CBG), [[cannabinol]] (CBN), [[cannabichromene]] (CBC), cannabidivarinic acid (CBDVA), cannabigerolic acid (CBGA), and Δ8-THC. | Two cannabinoids for which standards were obtained, [[cannabidivarin]] (CBDV) and [[cannabicyclol]] (CBL), were not detected in any strain. The 11 remaining cannabinoids with available chemical reference standards were identified and quantified. THCA content ranged from 0.76 to 20.71% w/w, with almost a linear increase in content from the lowest to highest strain with an r2 of 0.97, while CBDA content ranged from <MDL to 18.11% w/w, with the highest CBDA strains having the lowest THCA contents (Fig. 1). In THC-abundant strains, the CBDA levels were less than 0.15%, while in CBD abundant strains the content was greater than 5%. THC, the decarboxylated form of THCA, was present in strains from <LOQ up to 2% by weight in some strains, while CBD contents ranged from <MDL to 0.8%. CBD was most prevalent in high-CBDA strains. In addition, seven cannabinoids present at lower levels were quantified using individual calibration standards: [[tetrahydrocannabivarin]] (THCV), [[cannabigerol]] (CBG), [[cannabinol]] (CBN), [[cannabichromene]] (CBC), cannabidivarinic acid (CBDVA), cannabigerolic acid (CBGA), and Δ8-THC. | ||
[[File:Fig1 Mudge ScientificReports2018 8.png|1200px]] | |||
{{clear}} | |||
{| | |||
| STYLE="vertical-align:top;"| | |||
{| border="0" cellpadding="5" cellspacing="0" width="1200px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Fig. 1''' Biosynthetic pathway of cannabinoids originating from olivetolic acid and geranyl pyrophosphate. Graphs describe the cannabinoid contents within the 33 strains obtained arranged from lowest to highest total THC.</blockquote> | |||
|- | |||
|} | |||
|} | |||
===Classification of strains=== | |||
We hypothesized that individual plant breeders selected for cannabis strains by up-regulating and down-regulating specific enzymes within the biosynthetic pathways, resulting in a redirection of metabolites between THCA and CBDA. Our data analysis identified five clusters of strains that fall within a narrow range of total CBD/THC values consistent with this hypothesis (Table 1). The branch of the biosynthetic pathway with olivetolic acid and geranyl pyrophosphate as precursors produces CBGA, CBG, CBCA, CBC, THCA, THC, CBDA and CBD (Fig. 1). Strains from all clusters contained measurable amounts of CBGA, CBG, THCA and THC (Fig. 1). Nine strains from the clusters with higher concentrations of THCA (blue and purple) did not contain detectable levels of CBC (Fig. 1). Two of the clusters were not found to contain significant quantities of CBDA and CBD (Fig. 1; blue and purple). One strain was different from all others and had a greater CBDA content and detectable levels of CBGA, CBG, CBC, and CBD with minimal THCA and THC (Fig. 1; red). | |||
{| | |||
| STYLE="vertical-align:top;"| | |||
{| class="wikitable" border="1" cellpadding="5" cellspacing="0" width="80%" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" colspan="5"|'''Table 1.''' Strains of cannabis were clustered into five distinct groups that could be separated by the flow of metabolites through the CBD and THC pathways. | |||
|- | |||
|- | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;"|Group | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;"|Color code | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;"|CBD range (% w/w) | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;"|THC range (% w/w) | |||
! style="background-color:#e2e2e2; padding-left:10px; padding-right:10px;"|# of strains | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|A | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Blue | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|<MDL–0.08 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|11.3–19.1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|20 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|B | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Purple | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|<MDL–0.02 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|8.0–9.9 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|3 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|C | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Orange | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|7.1–9.7 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|5.0–6.7 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|6 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|D | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Green | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|5.3–8.8 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|1.7–3.1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|3 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|E | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Red | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|16.1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|0.7 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|1 | |||
|- | |||
|} | |||
|} | |||
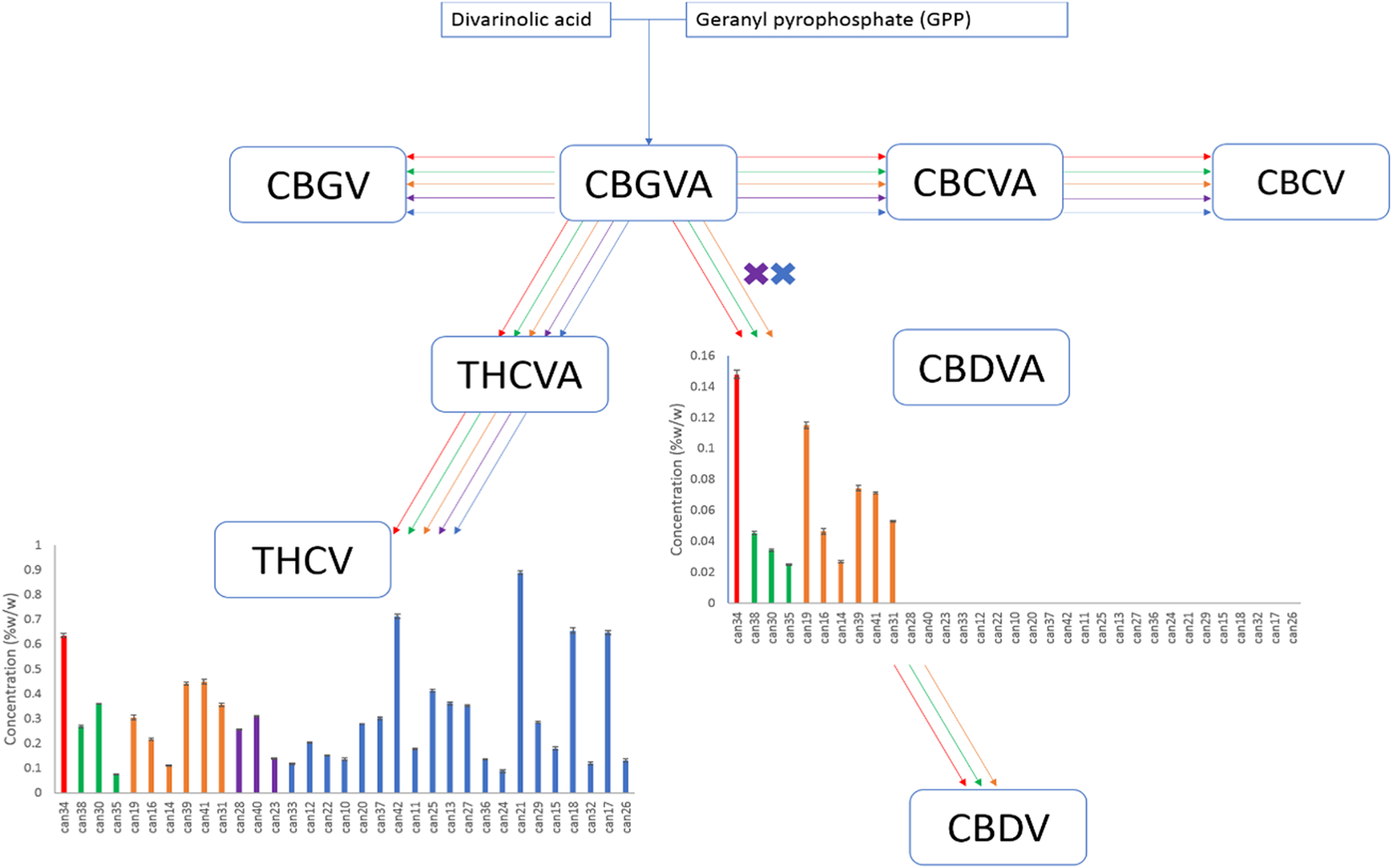
Compounds produced from the precursors divarinolic acid and geranyl pyrophosphate via cannabigerovarin acid (CBGVA) were also found to differ by strain cluster (Fig. 2). CBGVA appears to be a branch point for allocation of resources in cannabis between THCV and CBDVA, indicating that the enzyme activity or the resource allocation mechanism for production of THCV was lost in the breeding process of strains clustered in the red, orange, and green groups (Fig. 2). | |||
[[File:Fig2 Mudge ScientificReports2018 8.png|1000px]] | |||
{{clear}} | |||
{| | |||
| STYLE="vertical-align:top;"| | |||
{| border="0" cellpadding="5" cellspacing="0" width="1000px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Fig. 2''' Biosynthetic pathway of cannabinoids originating from divarinolic acid and geranyl pyrophosphate. Graphs describe the cannabinoid contents within the 33 strains obtained arranged from lowest to highest total THC.</blockquote> | |||
|- | |||
|} | |||
|} | |||
===Untargeted metabolomics analysis=== | |||
In addition to the 11 cannabinoids that corresponded with authentic standards, 21 peaks were identified in the [[Chromatography|chromatograms]] with UV spectra characteristic of cannabinoids. By comparison to THC, the contents were estimated from <MDL up to 0.34% by weight. Two unknown cannabinoids (CMPD-7 and CMPD-11) were detected in all strains, while CMPD-3 and CMPD-20 were each only detected in a single strain. | |||
===Relationships between known and unknown cannabinoids=== | |||
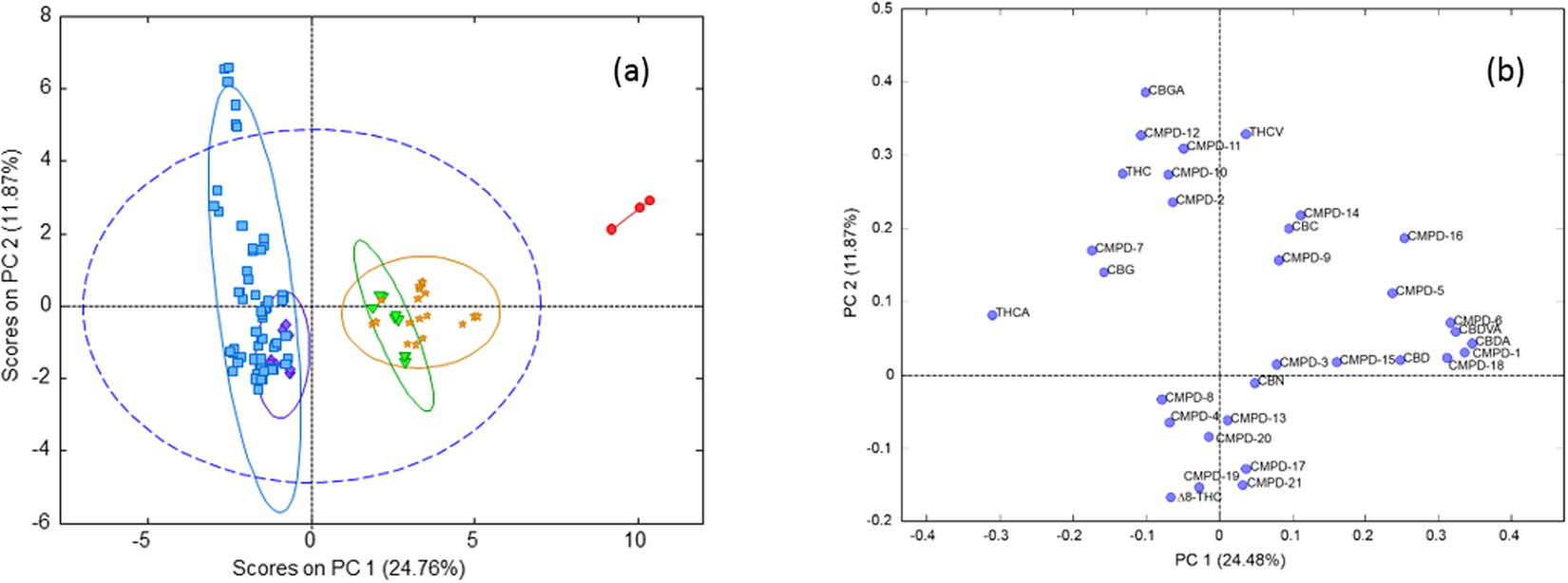
A principal component analysis (PCA) of the autoscaled cannabinoid data was plotted to show the clustering of the samples in an unsupervised fashion (Fig. 3). In the PCA plot, the first two principal components (PC) captured 36.6% of the variance in the data. Based on the loadings plot, the first PC was most highly influenced by the THCA and CBDA content of the strains, which are negatively correlated. There are two high THC strains (CAN17 and CAN21) and one CBD strain (CAN34) that were separated from the data clustered within the 95% confidence limit of the total data variance. Based on the loadings plot (Fig. 3B), CAN17 and CAN21 may be influenced by a significant number of low abundance cannabinoids including CBGA, CMPD-12, and CMPD-11. CAN34 is likely due to its significantly higher CBDA content relative to the other strains and because it contained less than 1% total THC. | |||
[[File:Fig3 Mudge ScientificReports2018 8.png|1000px]] | |||
{{clear}} | |||
{| | |||
| STYLE="vertical-align:top;"| | |||
{| border="0" cellpadding="5" cellspacing="0" width="1000px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Fig. 3''' Principal Component Analysis (PCA) of cannabinoid profiles classified according to THC/CBD contents ('''a''') scores plot ('''b''') loadings plot.</blockquote> | |||
|- | |||
|} | |||
|} | |||
==References== | ==References== | ||
Revision as of 20:52, 28 May 2019
| Full article title | Chemometric analysis of cannabinoids: Chemotaxonomy and domestication syndrome |
|---|---|
| Journal | Scientific Reports |
| Author(s) | Mudge, E.M.; Murch, S.J.; Brown, P.N. |
| Author affiliation(s) | University of British Columbia, British Columbia Institute of Technology |
| Primary contact | Email: Send message through journal website |
| Year published | 2018 |
| Volume and issue | 8 |
| Page(s) | 13090 |
| DOI | 10.1038/s41598-018-31120-2 |
| ISSN | 2045-2322 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.nature.com/articles/s41598-018-31120-2 |
| Download | https://www.nature.com/articles/s41598-018-31120-2.pdf (PDF) |
|
|
This article should not be considered complete until this message box has been removed. This is a work in progress. |
Abstract
Cannabis is an interesting domesticated crop with a long history of cultivation and use. Strains have been selected through informal breeding programs with undisclosed parentage and criteria. The term “strain” refers to minor morphological differences and grower branding rather than distinct cultivated varieties. We hypothesized that strains sold by different licensed producers are chemotaxonomically indistinguishable and that the commercial practice of identifying strains by the ratio of total Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) is insufficient to account for the reported human health outcomes. We used targeted metabolomics to analyze 11 known cannabinoids and an untargeted metabolomics approach to identify 21 unknown cannabinoids. Five clusters of chemotaxonomically indistinguishable strains were identified from the 33 commercial products. Only three of the clusters produce cannabidiolic acid (CBDA) in significant quantities, while the other two clusters redirect metabolic resources toward the tetrahydrocannabinolic acid (THCA) production pathways. Six unknown metabolites were unique to CBD-rich strains and/or correlated to CBDA, and three unknowns were found only in THC-rich strains. Together, these data indicate the domestication of the Cannabis germplasm has resulted in a loss of the CBDA pathway in some strains and reallocation of resources between CBDA and THCA pathways in others. The impact of domestication is a lack of chemical diversity and loss of biodiversity in modern Cannabis strains.
Introduction
Cannabis sativa L. (marijuana) is a dioecious, annual plant from Central Asia that has been used medicinally and recreationally for thousands of years.[1] The domestication of Cannabis has included human selection, inbreeding, and cross breeding, as well as natural outcrossing and genome mixing.[1] Strains are not easily delineated by genotype, and only moderate correlations have been observed between C. indica and C. sativa ancestry. In addition, large genetic variance has been observed within identically named strains.[2][3] Standardized, highly controlled programs to breed elite varieties or cultivars by selection of phytochemical profile have been limited.[4][5] It is estimated that there are several hundred or perhaps thousands of strains of cannabis currently being cultivated in legal and illegal markets.[4] It is possible that chemically identical or very closely related plant material is being sold under several different names by different producers, with no clear definition of the concept of a “strain.”
Cannabis producers market their products based on the amounts of total THC and CBD with the assumption that the overall phytochemical composition of the material can be extrapolated from these values, but there is considerable anecdotal evidence suggesting that strains with similar THC/CBD content have different effects on human physiology.[6][7] More than 120 different cannabinoids have been described in Cannabis[8][9], with the most interesting phytochemistry found in the glandular trichomes on the flowers of the female inflorescences.[10] THC is the most researched cannabinoid, and there are 10 additional classes of cannabinoids with varying chemical structures.[8] Cannabinoids are synthesized in acidic forms through the condensation of geranyl diphosphate (GPP) and most commonly olivetolic acid, products of the methylerythritol phosphate (MEP) and polyketide pathways.[11][12] There are several other polyketides that can be used in place of olivetolic acid, which contribute to the wide variation within this chemical class.[13][14] Neutral cannabinoids are products of decarboxylation from processing and handling harvested flowers.
Chemometric models are used to evaluate metabolite datasets to delineate relationships and identify potential influences on phytochemical diversity.[15][16][17] These approaches can be classified as targeted analysis, untargeted phytochemical discovery, metabolomic profiling. or fingerprinting.[15] Targeted metabolomics determines differences in known phytochemicals, while the untargeted approaches evaluate unidentified compounds in the phytochemical profiles.[15] Targeted-untargeted approaches combine known metabolites with the untargeted datasets as a hypothesis-generating tool to discover metabolite relationships, clusters, families and biochemical pathways.[15][18] The use of these models and algorithms enables a better understanding of metabolite commonality and diversity within plant species.[19]
We hypothesized that the total THC and CBD content is not sufficient to distinguish strains and that a combination of targeted and untargeted chemometric approaches can be used to predict cannabinoid composition and to better understand the impact of informal breeding program and selection on the phytochemical diversity of cannabis. To investigate these hypotheses, we assembled a collection of cannabis strains sold by licensed producers in Canada primarily based on total THC/CBD content, and analyzed the strains for known cannabinoids using a previously validated analytical method[20] to establish clusters of similar plant materials.
We then used an untargeted metabolomics approach to identify previously uncharacterized compounds and chemical relationships. We identified five clusters of chemotaxonomically indistinguishable strains within the collection. Our results show that the variation in less abundant cannabinoids between cannabis strains was not dependent on the total THC and CBD content. These data suggest that the domestication of the cannabis germplasm has resulted in the loss of the CBDA pathway in some strains and the reallocation of resources between CBDA and THCA pathways in others.
Results
Targeted metabolomics of cannabinoids
Two cannabinoids for which standards were obtained, cannabidivarin (CBDV) and cannabicyclol (CBL), were not detected in any strain. The 11 remaining cannabinoids with available chemical reference standards were identified and quantified. THCA content ranged from 0.76 to 20.71% w/w, with almost a linear increase in content from the lowest to highest strain with an r2 of 0.97, while CBDA content ranged from <MDL to 18.11% w/w, with the highest CBDA strains having the lowest THCA contents (Fig. 1). In THC-abundant strains, the CBDA levels were less than 0.15%, while in CBD abundant strains the content was greater than 5%. THC, the decarboxylated form of THCA, was present in strains from <LOQ up to 2% by weight in some strains, while CBD contents ranged from <MDL to 0.8%. CBD was most prevalent in high-CBDA strains. In addition, seven cannabinoids present at lower levels were quantified using individual calibration standards: tetrahydrocannabivarin (THCV), cannabigerol (CBG), cannabinol (CBN), cannabichromene (CBC), cannabidivarinic acid (CBDVA), cannabigerolic acid (CBGA), and Δ8-THC.
|
Classification of strains
We hypothesized that individual plant breeders selected for cannabis strains by up-regulating and down-regulating specific enzymes within the biosynthetic pathways, resulting in a redirection of metabolites between THCA and CBDA. Our data analysis identified five clusters of strains that fall within a narrow range of total CBD/THC values consistent with this hypothesis (Table 1). The branch of the biosynthetic pathway with olivetolic acid and geranyl pyrophosphate as precursors produces CBGA, CBG, CBCA, CBC, THCA, THC, CBDA and CBD (Fig. 1). Strains from all clusters contained measurable amounts of CBGA, CBG, THCA and THC (Fig. 1). Nine strains from the clusters with higher concentrations of THCA (blue and purple) did not contain detectable levels of CBC (Fig. 1). Two of the clusters were not found to contain significant quantities of CBDA and CBD (Fig. 1; blue and purple). One strain was different from all others and had a greater CBDA content and detectable levels of CBGA, CBG, CBC, and CBD with minimal THCA and THC (Fig. 1; red).
| |||||||||||||||||||||||||||||||||||
Compounds produced from the precursors divarinolic acid and geranyl pyrophosphate via cannabigerovarin acid (CBGVA) were also found to differ by strain cluster (Fig. 2). CBGVA appears to be a branch point for allocation of resources in cannabis between THCV and CBDVA, indicating that the enzyme activity or the resource allocation mechanism for production of THCV was lost in the breeding process of strains clustered in the red, orange, and green groups (Fig. 2).
|
Untargeted metabolomics analysis
In addition to the 11 cannabinoids that corresponded with authentic standards, 21 peaks were identified in the chromatograms with UV spectra characteristic of cannabinoids. By comparison to THC, the contents were estimated from <MDL up to 0.34% by weight. Two unknown cannabinoids (CMPD-7 and CMPD-11) were detected in all strains, while CMPD-3 and CMPD-20 were each only detected in a single strain.
Relationships between known and unknown cannabinoids
A principal component analysis (PCA) of the autoscaled cannabinoid data was plotted to show the clustering of the samples in an unsupervised fashion (Fig. 3). In the PCA plot, the first two principal components (PC) captured 36.6% of the variance in the data. Based on the loadings plot, the first PC was most highly influenced by the THCA and CBDA content of the strains, which are negatively correlated. There are two high THC strains (CAN17 and CAN21) and one CBD strain (CAN34) that were separated from the data clustered within the 95% confidence limit of the total data variance. Based on the loadings plot (Fig. 3B), CAN17 and CAN21 may be influenced by a significant number of low abundance cannabinoids including CBGA, CMPD-12, and CMPD-11. CAN34 is likely due to its significantly higher CBDA content relative to the other strains and because it contained less than 1% total THC.
|
References
- ↑ 1.0 1.1 Clarke, R.C.; Merlin, M.D. (2016). "Cannabis Domestication, Breeding History, Present-day Genetic Diversity, and Future Prospects". Critical Reviews in Plant Sciences 35 (5–6): 293–327. doi:10.1080/07352689.2016.1267498.
- ↑ Sawler, J.; Stout, J.M.; Fardner, K.M. et al. (2015). "The Genetic Structure of Marijuana and Hemp". PLoS One 10 (8): e0133292. doi:10.1371/journal.pone.0133292. PMC PMC4550350. PMID 26308334. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC4550350.
- ↑ Soler, S.; Gramazio, P.; Figàs, M.R. et al. (2017). "Genetic structure of Cannabis sativa var. indica cultivars based on genomic SSR (gSSR) markers: Implications for breeding and germplasm management". Industrial Crops and Products 104: 171–78. doi:10.1016/j.indcrop.2017.04.043.
- ↑ 4.0 4.1 Small, E. (2015). "Evolution and Classification of Cannabis sativa (Marijuana, Hemp) in Relation to Human Utilization". The Botanical Review 81 (3): 189–294. doi:10.1007/s12229-015-9157-3.
- ↑ de Meijer, E. (2014). "Chapter 5: The Chemical Phenotypes (Chemotypes) of Cannabis". In Pertwee, R.. Handbook of Cannabis. Oxford Scholarship Online. pp. 89–110. doi:10.1093/acprof:oso/9780199662685.003.0005. ISBN 9780199662685.
- ↑ McPartland, J.M.; Russo, E.B. (2001). "Cannabis and Cannabis Extracts". Journal of Cannabis Therapeutics 1 (3–4): 103–32. doi:10.1300/J175v01n03_08.
- ↑ Russo, E.B. (2011). "Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects". British Journal of Pharmacology 163 (7): 1344–64. doi:10.1111/j.1476-5381.2011.01238.x. PMC PMC3165946. PMID 21749363. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC3165946.
- ↑ 8.0 8.1 ElSohly, M.a.; Gul, W. (2014). "Chapter 1: Constituents of Cannabis sativa". In Pertwee, R.. Handbook of Cannabis. Oxford Scholarship Online. pp. 3–22. doi:10.1093/acprof:oso/9780199662685.003.0001. ISBN 9780199662685.
- ↑ Turner, C.E.; ElSohly, M.A.; Boeren, E.G. (1980). "Constituents of Cannabis sativa L. XVII. A review of the natural constituents". Journal of Natural Products 43 (2): 169-234. PMID 6991645.
- ↑ Turner, J.C.; Hemphill, J.K.; Mahlberg, P.G. (1978). "Quantitative determination of cannabinoids in individual glandular trichomes of Cannabis sativa L. (Cannabacaea)". American Journal of Botany 65 (10): 1103–06. doi:10.1002/j.1537-2197.1978.tb06177.x.
- ↑ Flores-Sanchez, I.J.; Verpoorte, R. (2008). "Secondary metabolism in cannabis". Phytochemistry Reviews 7 (3): 615–639. doi:10.1007/s11101-008-9094-4.
- ↑ Fellermeier, M.; Eisenreich, W.; Bacher, A. et al. (2001). "Biosynthesis of cannabinoids. Incorporation experiments with (13)C-labeled glucoses". European Journal of Biochemistry 268 (6): 1596-604. PMID 11248677.
- ↑ Degenhardt, F.; Stehle, F.; Kayser, O. (2017). "Chapter 2: The Biosynthesis of Cannabinoids". In Preedy, V.R.. Handbook of Cannabis and Related Pathologies. Elsevier. pp. 13–23. doi:10.1016/B978-0-12-800756-3.00002-8. ISBN 9780128007563.
- ↑ Shoyama, Y.; Hirano, H.; Nishioka, I. (1984). "Biosynthesis of propyl cannabinoid acid and its biosynthetic relationship with pentyl and methyl cannabinoid acids". Phytochemistry 23 (9): 1909–12. doi:10.1016/S0031-9422(00)84939-0.
- ↑ 15.0 15.1 15.2 15.3 Turi, C.E.; Finley, J.; Shipley, P.R. et al. (2015). "Metabolomics for phytochemical discovery: development of statistical approaches using a cranberry model system". Journal of Natural Products 78 (4): 953-66. doi:10.1021/np500667z. PMID 25751407.
- ↑ Worley, B.; Powers, R. (2013). "Multivariate Analysis in Metabolomics". Current Metabolomics 1 (1): 92–107. doi:10.2174/2213235X11301010092. PMC PMC4465187. PMID 26078916. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC4465187.
- ↑ Hagel, J.M.; Facchini, P.J. (2008). "Plant metabolomics: analytical platforms and integration with functional genomics". Phytochemistry Reviews 7 (3): 479–497. doi:10.1007/s11101-007-9086-9.
- ↑ Brown, P.N;. Murch, S.J.; Shipley, P. (2012). "Phytochemical diversity of cranberry (Vaccinium macrocarpon Aiton) cultivars by anthocyanin determination and metabolomic profiling with chemometric analysis". Journal of Agricultural and Food Chemistry 60 (1): 261–71. doi:10.1021/jf2033335. PMID 22148867.
- ↑ Scherling, C.; Roscher, C.; Giavalisco, P. et al. (2010). "Metabolomics unravel contrasting effects of biodiversity on the performance of individual plant species". PLoS One 5 (9): e12569. doi:10.1371/journal.pone.0012569. PMC PMC2935349. PMID 20830202. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC2935349.
- ↑ Mudge, E.M.; Murch, S.J.; Brown, P.N. (2017). "Leaner and greener analysis of cannabinoids". Analytical and Bioanalytical Chemistry 409 (12): 3153–63. doi:10.1007/s00216-017-0256-3. PMC PMC5395585. PMID 28233028. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC5395585.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added.