Difference between revisions of "Template:Article of the month"
From CannaQAWiki
Jump to navigationJump to searchShawndouglas (talk | contribs) (Updated article of the month text.) |
Shawndouglas (talk | contribs) |
||
| Line 2: | Line 2: | ||
'''"[[Journal:An assessment of heavy metal contaminants related to cannabis-based products in the South African market|An assessment of heavy metal contaminants related to cannabis-based products in the South African market]]"''' | '''"[[Journal:An assessment of heavy metal contaminants related to cannabis-based products in the South African market|An assessment of heavy metal contaminants related to cannabis-based products in the South African market]]"''' | ||
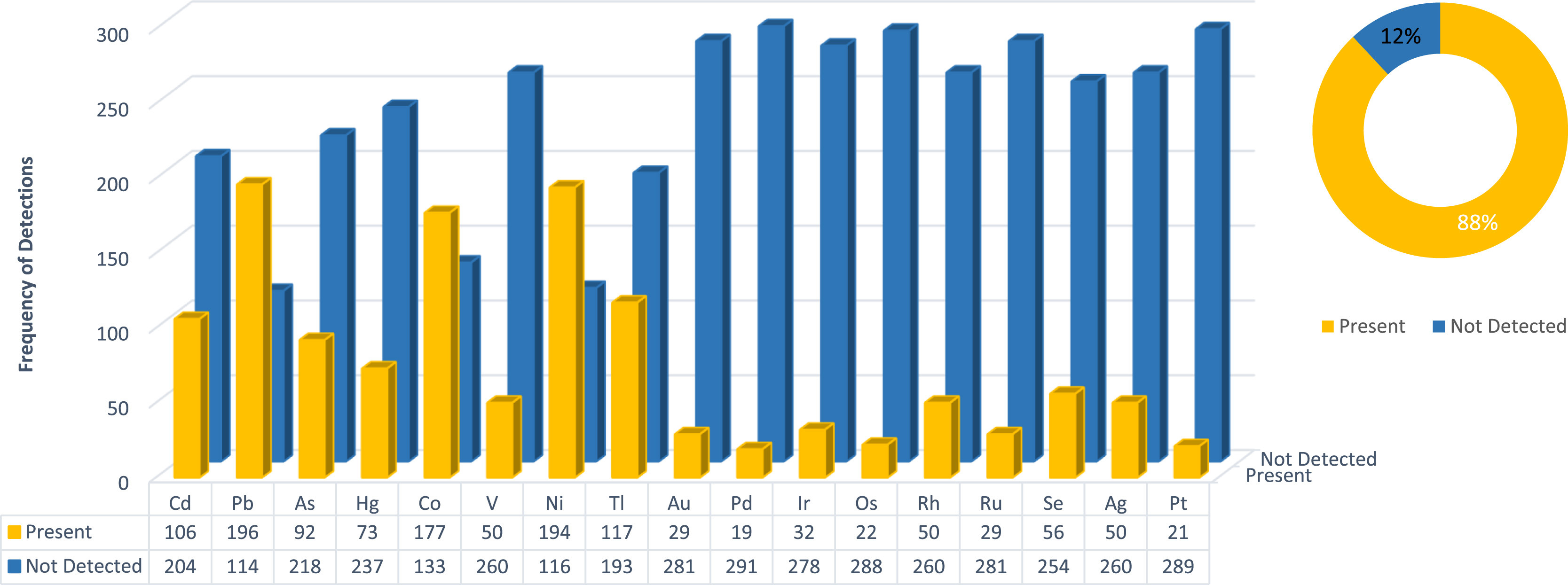
South African [[cannabis]]-based products that were submitted to a private [[laboratory]] for the determination of [[heavy metals]] residues were analyzed. The presence of each heavy metal residue was determined in order to establish which residues are most prevalent in [[Sample (material)|samples]]. Two specifications were considered for both oral as well as inhalation limits: ''[[United States Pharmacopeia]]'' (''USP'') <232>/<233> and the [[International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use]] (ICH) Q3D. To date, no data of this kind exist in South Africa specifically relating to cannabis-based [[Cannabis (drug)|medicinal]], recreational, or complementary products. | South African [[cannabis]]-based products that were submitted to a private [[laboratory]] for the determination of [[heavy metals]] residues were analyzed. The presence of each heavy metal residue was determined in order to establish which residues are most prevalent in [[Sample (material)|samples]]. Two specifications were considered for both oral as well as inhalation limits: ''[[United States Pharmacopeia]]'' (''USP'') <232>/<233> and the [[International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use]] (ICH) Q3D. To date, no data of this kind exist in South Africa specifically relating to cannabis-based [[Cannabis (drug)|medicinal]], recreational, or complementary products ... ('''[[Journal:An assessment of heavy metal contaminants related to cannabis-based products in the South African market|Full article...]]''')<br /> | ||
<br /> | <br /> | ||
Revision as of 00:11, 30 November 2021
South African cannabis-based products that were submitted to a private laboratory for the determination of heavy metals residues were analyzed. The presence of each heavy metal residue was determined in order to establish which residues are most prevalent in samples. Two specifications were considered for both oral as well as inhalation limits: United States Pharmacopeia (USP) <232>/<233> and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q3D. To date, no data of this kind exist in South Africa specifically relating to cannabis-based medicinal, recreational, or complementary products ... (Full article...)
Recently featured:
- ▪ Essential oil of Cannabis sativa L: Comparison of yield and chemical composition of 11 hemp genotypes
- ▪ Risk assessment of over-the-counter cannabinoid-based cosmetics: Legal and regulatory issues governing the safety of cannabinoid-based cosmetics in the UAE
- ▪ Metabolomic analysis of cannabinoid and essential oil profiles in different hemp (Cannabis sativa L.) phenotypes