Pinitol
| |
| Names | |
|---|---|
| IUPAC name
1D-chiro-Inositol
| |
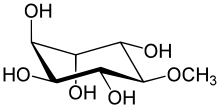
| Systematic IUPAC name
(1R,2S,3R,4S,5S,6S)-6-Methoxycyclohexane-1,2,3,4,5-pentol | |
| Other names
3-O-Methyl-D-chiro-inositol
D-(+)-chiro-Inositol D-Pinitol Inzitol D-(+)-Pinitol (+)-Pinitol Sennitol Pinnitol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H14O6 | |
| Molar mass | 194.183 g·mol−1 |
| Melting point | 179 to 185 °C (354 to 365 °F; 452 to 458 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pinitol is a cyclitol, a cyclic polyol. It is a known anti-diabetic agent isolated from Sutherlandia frutescens leaves.[1][2] Gall plant tannins can be differentiated by their content of pinitol.[3] It was first identified in the sugar pine (Pinus lambertiana).[4] It is also found in other plants, such as in the pods of the carob tree.[5]
Certain variants of the bacteria Pseudomonas putida have been used in organic synthesis, the first example being the oxidation of benzene, employed by Steven Ley in the synthesis of (±)-pinitol.[6]
Glycosides
Ciceritol is a pinitol digalactoside that can be isolated from seeds of chickpea, lentil and white lupin.[7]
A cyclitol derivative can be found in the marine sponge Petrosia sp.[8]
Biosynthesis
D-pinitol is the most widely distributed inositol ether in plants.[9] In Angiosperms, D-pinitol has a relatively straight forward and short biosynthesis which proceeds via the Loewus pathway. The precursor to the biosynthesis pathway is glucose-6-phosphate, which is converted to D-ononitol (1-D-4-O-methyl-myo-inositol) via myo-inositol. Ononitol is epimerized to yield D-pinitol via a D-ononitol epimerase using NADPH as a cofactor.[10]

References
- ^ Narayanan CR, Joshi DD, Mujumdar AM, Dhekne VV (1987). "Pinitol—A new anti-diabetic compound from the leaves of Bougainvillea spectabilis" (PDF). Current Science. 56 (3): 139–141. JSTOR 24091051.
- ^ "Introduction Sutherlandia frutesoens—Kankerbossie" (PDF). Afrikaanse Kruiden. 2005-08-04. Archived from the original (PDF) on 2006-09-14.
- ^ Sanz ML, Martínez-Castro I, Moreno-Arribas MV (2008). "Identification of the origin of commercial enological tannins by the analysis of monosaccharides and polyalcohols". Food Chemistry. 111 (3): 778–783. doi:10.1016/j.foodchem.2008.04.050. S2CID 84922451.
- ^ Anderson AB, MacDonald DL, Fischer HO (1952). "The structure of pinitol". Journal of the American Chemical Society. 74 (6): 1479–1480. doi:10.1021/ja01126a036. S2CID 101698212.
- ^ Tetik N, Yüksel E (March 2014). "Ultrasound-assisted extraction of D-pinitol from carob pods using Response Surface Methodology". Ultrasonics Sonochemistry. 21 (2): 860–865. doi:10.1016/j.ultsonch.2013.09.008. PMID 24090831. S2CID 28123933.
- ^ Ley SV, Sternfeld F, Taylor S (1987). "Microbial oxidation in synthesis: A six step preparation of (±)-pinitol from benzene". Tetrahedron Letters. 28 (2): 225–226. doi:10.1016/S0040-4039(00)95692-2. S2CID 83944164.
- ^ Quemener B, Brillouet JM (1983). "Ciceritol, a pinitol digalactoside from seeds of chickpea, lentil and white lupin". Phytochemistry. 22 (8): 1745–1751. doi:10.1016/S0031-9422(00)80263-0. S2CID 84765529.
- ^ Kim DK, Lim YJ, Kim JS, Park JH, Kim ND, Im KS, et al. (May 1999). "A cyclitol derivative as a replication inhibitor from the marine sponge Petrosia sp". Journal of Natural Products. 62 (5): 773–776. doi:10.1021/np9804785. PMID 10346968. S2CID 20297208.
- ^ Sánchez-Hidalgo M, León-González AJ, Gálvez-Peralta M, González-Mauraza NH, Martin-Cordero C (2021-02-01). "d-Pinitol: a cyclitol with versatile biological and pharmacological activities". Phytochemistry Reviews. 20 (1): 211–224. doi:10.1007/s11101-020-09677-6. ISSN 1572-980X.
- ^ Dumschott K, Dechorgnat J, Merchant A (May 2019). "Water Deficit Elicits a Transcriptional Response of Genes Governing d-pinitol Biosynthesis in Soybean (Glycine max)". International Journal of Molecular Sciences. 20 (10): 2411. doi:10.3390/ijms20102411. PMC 6566849. PMID 31096655.
External links
Notes
This article is a direct transclusion of the Wikipedia article and therefore may not meet the same editing standards as CannabisQAwiki.