Journal:Essential oil of Cannabis sativa L: Comparison of yield and chemical composition of 11 hemp genotypes
| Full article title | Essential oil of Cannabis sativa L: Comparison of yield and chemical composition of 11 hemp genotypes |
|---|---|
| Journal | Molecules |
| Author(s) | Pieracci, Yienia; Ascrizzi, R.; Terreni, Valentina; Pistelli, Luisa; Flamini, Guido; Bassolino, Laura; Fulvio, Flavia; Montanari, Massimo; Paris, Roberta |
| Author affiliation(s) | University of Pisa, CREA – Cereal and Industrial Crop Research Centre, University of Foggia |
| Primary contact | Email: roberta dot ascrizzi at gmail dot com |
| Year published | 2021 |
| Volume and issue | 26(13) |
| Article # | 4080 |
| DOI | 10.3390/molecules26134080 |
| ISSN | 1420-3049 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.mdpi.com/1420-3049/26/13/4080/htm |
| Download | https://www.mdpi.com/1420-3049/26/13/4080/pdf (PDF) |
Abstract
Cannabis sativa L. is an annual species cultivated since antiquity for different purposes. While in the past hemp inflorescences were considered crop residues, at present they are regarded as valuable raw materials with different applications, among which extraction of the essential oil (EO) has gained increasing interest in many fields. The aim of the present study is the evaluation of the yield and the chemical composition of the EO obtained by hydrodistillation from 11 hemp genotypes, cultivated in the same location for two consecutive growing seasons. The composition of the EOs was analyzed by gas chromatography–mass spectrometry (GC–MS) and then subjected to multivariate statistical analysis. Sesquiterpenes represented the main class of compounds in all the EOs, both in their hydrocarbon and oxygenated forms, with relative abundances ranging from 47.1 to 78.5%; the only exception was the Felina 32 sample collected in 2019, in which cannabinoids predominated. Cannabinoids were the second most abundant class of compounds, of which cannabidiol was the main one, with relative abundances between 11.8 and 51.5%. The statistical distribution of the samples, performed on the complete chemical composition of the EOs, evidenced a partition based on the year of cultivation, rather than on the genotype, with the exception of Uso-31. Regarding the extraction yield, a significant variation was evidenced among both the genotypes and the years of cultivation.
Keywords: monoecious, dioecious, by-products, monoterpenes, sesquiterpenes, cannabinoids, flowering behavior, cannabidiol
Introduction
Cannabis sativa L. is an annual herb belonging to the Cannabaceae family, which has been cultivated since antiquity as a source of fiber, seed oil, food, and medicine, as well as for recreational and religious purposes.[1] It has evolved as a dioecious species, with female and male flowers on different individuals, but selection processes have led to the development of monoecious genotypes that bare male and female flowers on the same individual. Thus, depending on the intended use, the morphology of the plants varies significantly between genotypes in terms of height, biomass, and seed yield.[1][2]
The female inflorescences and leaves of the Cannabis plant are covered in glandular trichomes, which are considered biofactories of phytochemicals[3] due to their ability to synthesize and store different secondary metabolites, of which phytocannabinoids are the best known and studied.[4] On the basis of their cannabinoid content, in particular of their cannabidiol (CBD)/tetrahydrocannabinol (THC) ratio, Cannabis sativa L. genotypes are divided into five distinguished chemical phenotypes: (i) chemotype I, or drug-type (with the predominant cannabinoid being THC); (ii) chemotype II, or intermediate-type (with the predominant cannabinoids being CBD and THC); (iii) chemotype III or fiber-type (with the predominant cannabinoid being CBD); chemotype IV (with a prevalence of [[cannabigerol] [CBG]); and chemotype V, classifying materials with undetectable amounts of any cannabinoid.[5][6]
Since 2001, when the regulatory European Commission (E.C.) No. 2860/2000 entered into force, the European Union (E.U.) authorized the cultivation of hemp complying with the 0.2% w/w Δ9-THC threshold.[7] As a consequence, hemp cultivation for fiber and seed production was resumed, and more attention was paid to agro-industrial waste of the hemp chain, among which inflorescences, as valuable sources of bioactive molecules to feed the pharmaceutical, cosmeceutical, and manufacturing industries, in the perspective of the sustainable circular economy. In recent years, the extraction of hemp-based essential oil (EO) has gained increasing interest as a value-added product[8], thanks to its various fields of application.[9] Hemp EO showed its best outcomes as an environmentally friendly insecticide against aphids, housefly populations, and mosquitoes[10], as a noteworthy toxic effect against Aedes albopictus is reported. Moreover, it exerts positive toxic activity towards the snail Physella acuta, an intermediate host of nematode and trematode human parasites, as well as being a common disease for rice fields.[11] In the agricultural field, hemp EO exhibits strong allelopathic activity against invasive weed germination, as well as seedling growth.[12] Interestingly, EOs were reported to be effective against dermatophyte species, thus exerting a role in preventing skin disorders.[13] Moreover, its use as a beverage flavoring agent has been reported.[9]
Essential oil from Cannabis is a complex mixture of volatile compounds made up of more than 100 terpenes and terpenoids (the oxygen-containing terpenes), known as the major contributors of the peculiar aromatic profile of different Cannabis strains.[14][15][16] Monoterpenes (10 C) and sesquiterpenes (15 C) constitute the largest content of the hemp essential oil, in both their hydrocarbon and oxygenated forms[17][18], followed by diterpenes (20 C). Almost every compound identified in the EO has its own characteristic fragrance, and their combination is responsible for the unique aromatic bouquet of different strains[19], which can influence consumers’ preferences; generally, hemp varieties with high percentages of monoterpenes are considered more pleasant than those with high percentages of sesquiterpenes.[20] Several factors, such as genotype, flowering behavior (dioecy or monoecy), cultivation technique, plant density, stage of development at harvest, material processing, and storage conditions can influence the composition of hemp EO (chemical profile) and its extraction yield.[19][21][22][23][24] Monoterpenes could be present in higher quantities in the fresh material, while drying and storage could determine their loss, leading to the increment of the relative portion of sesquiterpenes. Environmental[8][21] and weather conditions also seem to be an important factor for both the EO composition and yield; indeed, dry conditions between flowering stage and seed maturity can prevent trichome damage and EO yield losses.[22]
All these factors affecting the yield and chemical composition of hemp essential oils—including the lack of standardization of growing and operating conditions—make it hard to compare the chemical composition of the essential oils extracted from different Cannabis sativa L. genotypes in diverse studies. Given that, this study aimed to evaluate the EO yield and the volatile profile of 11 different hemp genotypes, both monoecious and dioecious, belonging to three chemotypes (III–V), and the differences that occurred during two years of cultivation, in order to promote their employment as value-added by-products based on their peculiar characteristics.
Results and discussions
Plant material features
Five monoecious (Uso-31, Carmaleonte, Codimono, Futura 75, and Felina 32) and six dioecious (Bernabeo, Carmagnola, CS, Fibranova, Fibrante, and Eletta Campana) European industrial hemp genotypes were cultivated in an open field for two consecutive years. The main characteristics of the cultivated plant material—including chemotypes, flower type, and flowering time—are reported in Table 1.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Sowing was performed on April 19, 2019 and April 23, 2020, with a 25 cm distance between rows on randomized blocks with three repetitions, and a plot size of 20 m2.
The harvest date differed according to the flower behavior and flowering time of each genotype. Generally, monoecious and early-flowering strains were harvested earlier than dioecious and late-flowering genotypes in both years (Table 1).
The comparison between plant heights showed differences between the two years, with higher plants obtained in 2020. The same trend was observed when comparing plant collar diameters. Plant density, defined as number of plants/m2, and dried inflorescences yield, measured as g/m2, were significantly lower in 2020, while dried inflorescences yield calculated for single plant was by far higher in 2020 than in 2019. These differences in plant growth in the two years of cultivation could be due to the lack of rainfall in the pre- and post-emergence period of 2020, compared to that of 2019, which could have caused a reduced germination rate and, therefore, a lower plant density. Conversely, 2019 was very rainy in May and June, favoring seed germination and following plant density. This rainfall trend was inverted in June; indeed, the rainy period (July and August) in 2020 could have allowed for a biomass production greater than that observed in 2019, as is clearly shown in Table 1. Finally, September 2019 was rainier than 2020, and this could have influenced the EOs’ yield.
Essential oil compositions and yields
The complete compositions and yields of the essential oils obtained from the dried inflorescences of 11 genotypes of Cannabis sativa L., cultivated by CREA-CI (Italy) in an experimental farm located in Rovigo and harvested during the years 2019 and 2020, are reported in Table 2 and Table 3, respectively. In total, 116 compounds were identified, representing 90.6–99.4% of the total composition.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
All the EOs, with the exception of Felina 32-2019, were characterized by a predominance of sesquiterpenes, in both their hydrocarbon and oxygenated forms, ranging from 47.1% in Carmagnola-2020 (Table 3) to 78.5% in Fibrante-2019 (Table 2). As reported in Table 4, both the hydrocarbon and oxygenated sesquiterpenes presented significant differences in all the EO compositions as a function of the genotype, the year of cultivation, and their interaction.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In 2019 EOs, sesquiterpene hydrocarbons exhibited a significantly higher relative concentration in Futura 75, followed by Fibrante and Carmagnola. Lower relative abundances were, instead, found in Bernabeo, Eletta Campana, and Felina 32 (Table 2). In 2020 samples, instead, this class of compounds was significantly more abundant in Carmaleonte, Codimono, Eletta Campana, Fibranova, and Futura 75, while their lowest presence was detected in Uso-31 (Table 3). Furthermore, sesquiterpene hydrocarbons were significantly higher in 2020 than in 2019 for each analyzed hemp genotype, except for Carmagnola and Uso-31. Among this chemical class, the main volatile compounds were β-caryophyllene and α-humulene, in accordance with Ascrizzi et al.[8], Vuerich et al.[18], and Menghini et al.[25], who reported these constituents as being typical of hemp varieties.
Regarding the oxygenated sesquiterpenes, among the 2019 EOs, Carmaleonte and Uso-31 presented higher relative abundances (59.6 and 60.9%, respectively), whilst the lowest was exhibited by Felina 32 (30.9%). Conversely to the hydrocarbon forms, oxygenated sesquiterpenes were significantly more abundant in all the 2019 samples, compared to those of 2020. These secondary metabolites are degradation products deriving from the oxidation of the corresponding terpenes due to air exposure (i.e., during prolonged storage), and are considered responsible for the antioxidant activity of many EOs.[18][26] Moreover, higher oxygenated compounds’ relative abundances reflect more favorable growth conditions, as was also confirmed by the higher plant density detected in the 2019 samples. The analyzed samples presented caryophyllene oxide and humulene oxide II as the main components of this chemical class, which were also reported as the main epoxides found in the hemp varieties analyzed by Micalizzi et al.[27] Furthermore, 14-hydroxy-9-epi-(E)-caryophyllene, reported by Ascrizzi at al.[8], was present in low relative abundances.
The amount of selinene derivative compounds (α-selinene, β-selinene, selina-3,7(11)-diene, and selin-6-en-4-ol) in 2019 and 2020 is notable, as this is a common occurrence in hemp EOs; their presence was more consistent in Futura 75, Eletta Campana, and CS, as has already been reported in the literature.[10][25][27] Bernabeo EOs in both years were characterized by the presence of higher percentages of neointermedeol, α-bulnesene, and juniper camphor; Fibrante EOs by trans-α-bergamotene and (E)-β-farnesene.
Monoterpenes were poorly represented in all the samples, with no distinction between monoecious and dioecious varieties, but with differences induced by the year of collection and the genotype. They accounted for up to 9.3% in Carmagnola 2020 (Table 3). Overall, nine hydrocarbons and 14 oxygenated monoterpenes were detected: among the former, α-pinene, β-pinene, and myrcene were the most representative, whilst in the latter, no compounds were revealed in appreciable relative abundances. Among the 2019 EOs, monoterpene hydrocarbons were significantly higher in Carmagnola and Fibrante (2.0 and 2.1%, respectively), and the latter presented a significantly higher content of oxygenated monoterpenes as well (1.3%). Fibrante-2019 also differed from the other genotypes in terms of its higher relative abundance in limonene (0.5%), which reached 0.8% in 2020. In the 2020 samples, hydrocarbon forms were found to be more abundant in Carmagnola (1.8%), while the oxygenated ones in CS (2.3%). However, with the exception of Codimono, 2020 samples were characterized by a significantly higher relative abundance of both hydrocarbon and oxygenated monoterpenes.
The amount of the compounds belonging to this class of secondary metabolites is very different from those reported by Bertoli et al.[21], Benelli et al.[10], and Nissen et al.[28], in which the main detected chemical class was represented by monoterpenes, with significant differences between monoecious and dioecious genotypes; in these papers, however, the starting material consisted of the fresh inflorescences, rather than the dried ones. Assuming that the monoterpene content decreased in the drying process and storage[26][27][29][30], the predominance of sesquiterpenes in the examined EOs might be due, at least in part, to the drying process, which might have induced some changes in the chemical composition of the starting material, such as (i) evaporation of the low boiling-point compounds[27], and (ii) the induction of oxidative reactions, as in the conversion of β-caryophyllene in caryophyllene oxide, and α-humulene in humulene oxide II.[30] Nevertheless, few of the literature studies used the dried hemp inflorescences for the collection and characterization of the EOs.
Finally, cannabinoids were the second relevant class of compounds in all the EOs, excluding that of Felina 32-2019, in which they were the main chemical group, accounting for 53.4% of the total (Table 2). The detected cannabinoids were cannabidiol, cannabichromene, and Δ9-tetrahydrocannabinol, but the first was the most abundant, with relative percentages ranging from 11.8 to 51.5% in Uso-31-2019 and Felina 32-2019, respectively (Table 2). In 2020 samples, these metabolites were detected in a greater relative amount in Carmagnola (37.7%) than in Bernabeo (15.0%, the lowest). In general, cannabinoids were meaningfully higher in the 2020 samples, except for Bernabeo and Felina 32, but according to the two-way ANOVA (Table 4), no significant differences were present between the two years of cultivation for this chemical class, which, on the contrary, showed significant differences among the genotypes. As for the sesquiterpenes, the predominance of cannabinoids in the EOs might be imputable to the drying process, during which the decarboxylation of cannabinoid acids into their relative volatile forms takes place.[30]
Nevertheless, all the detected classes of compound, excluding the already mentioned cannabinoids, presented significant differences as a function of genotype (G), year of cultivation (Y), and genotype–year interaction (G × Y), as evidenced by the two-way ANOVA (Table 4), which was also conducted on the chemical compounds, selected by the SIMPER test, which contributed to at least 1.00% of the dissimilarities in the composition between 2019 and 2020 EO samples. The statistical analysis evidenced that 16 compounds were responsible for an overall dissimilarity contribution of 32.94%, and only seven accounted for a total dissimilarity contribution above 55%. They comprised (i) the two main sesquiterpene hydrocarbons, β-caryophyllene and α-humulene; (ii) four oxygenated sesquiterpenes, caryophyllene oxide, 14-hydroxy-9-epi-(E)-caryophyllene, and the two isomers of caryophylla-4(14),8(15)-dien-5-ol; and (iii) one cannabinoid, cannabidiol. All the selected chemicals had significant differences based on the genotypes, year of cultivation, and their interaction.
The essential oil extraction yield ranged from 0.03 to 0.12% w/w for the samples collected in 2019, and from 0.03 to 0.23% w/w for the 2020 samples, where the highest yield was comparable to those already published in the literature. [20] A statistically significant difference in percentage was found among genotypes, year of cultivation, and the interaction between genotype and year (Table 4). Regarding the dependence of the EO extraction yield on the hemp genotype, several studies are present in the literature[18][19][22][28][31], while no references are available for its dependence on the year of cultivation.
For the 2019 samples, the significantly highest EO extraction yield was obtained by Futura 75 (0.12%), followed by Carmagnola (0.09%) and Eletta Campana (0.09%), while the lowest (0.03%) was from Bernabeo, Felina 32, and Uso-31. Regarding the 2020 samples, instead, Codimono, followed by Eletta Campana > CS > Fibrante (0.23% > 0.20% > 0.20% > 0.16%, respectively), were the most productive cultivars, whilst Uso-31 and Bernabeo were the least productive ones (0.03% and 0.05%, respectively). For Uso-31 (chemotype V), whose inflorescences are characterized by a negligible level of cannabinoids, the lowest EO extraction yield was found irrespective of the year. A positive correlation between the accumulation of total cannabinoids and total terpenes, both synthesized in glandular trichomes[22], has already been reported[32][33][34], which explains the low EO yield obtained for Uso-31. Considering both the years of cultivation, all the 2020 samples were characterized by a significantly higher EO extraction yield than the 2019 ones (Table 2 and Table 3), and this could be at least partly due to the different meteorological conditions occurring in the two subsequent growing seasons, particularly regarding rainfalls and average temperature (Table 5). Higher temperatures may indeed cause a higher spontaneous evaporation degree of the EO from the plant trichomes.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Multivariate statistical analysis
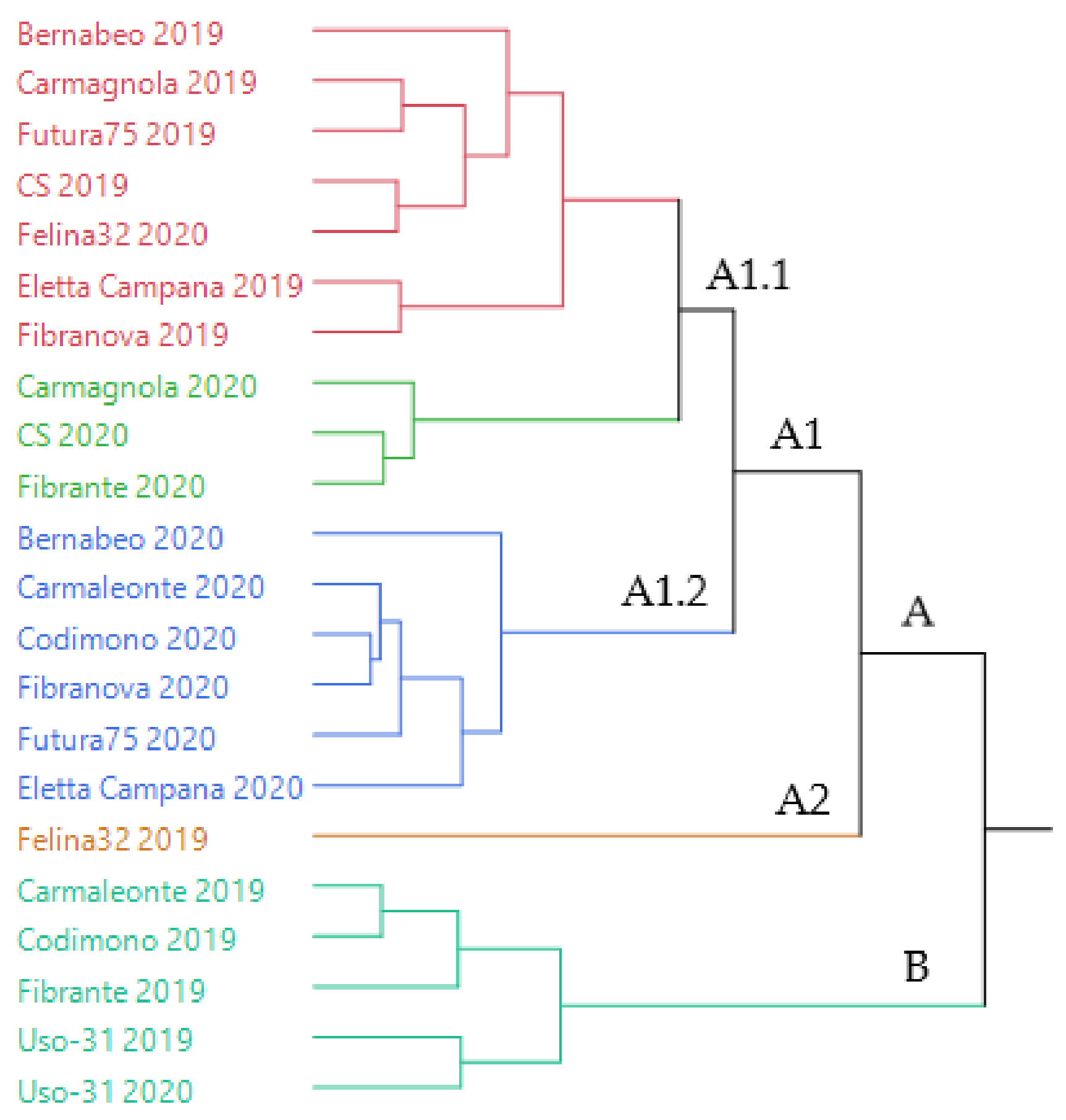
The dendrogram of the hierarchical cluster analysis (HCA) performed on the complete compositions of the EOs, extracted from all the samples of hemp of both years of cultivation, is reported in Figure 1.
|
The samples were distributed in two macro-clusters: A and B. Cluster A, the more heterogeneous, was divided in two sub-clusters, A1 and A2; A2 (orange) was formed of a single sample (Felina 32-2019), whilst A1 comprised two sub-groups, A1.1 and A1.2. The latter (evidenced in blue) was homogeneous, as it only included 2020 samples. Group A1.1, instead, resulted in two additional homogeneous groups (pink and green), of which the former mainly includes 2019 samples (except Felina 32-2020), while the latter only uses 2020 samples. Furthermore, cluster B (light blue) presented only one group, made up of 2019 samples, with the only exception of Uso-31.
The statistical distribution of the EOs samples evidenced a grouping based on the year of cultivation, rather than on the genotype, except for Uso-31, whose EOs of 2019 and 2020 occurred closely in the same cluster.
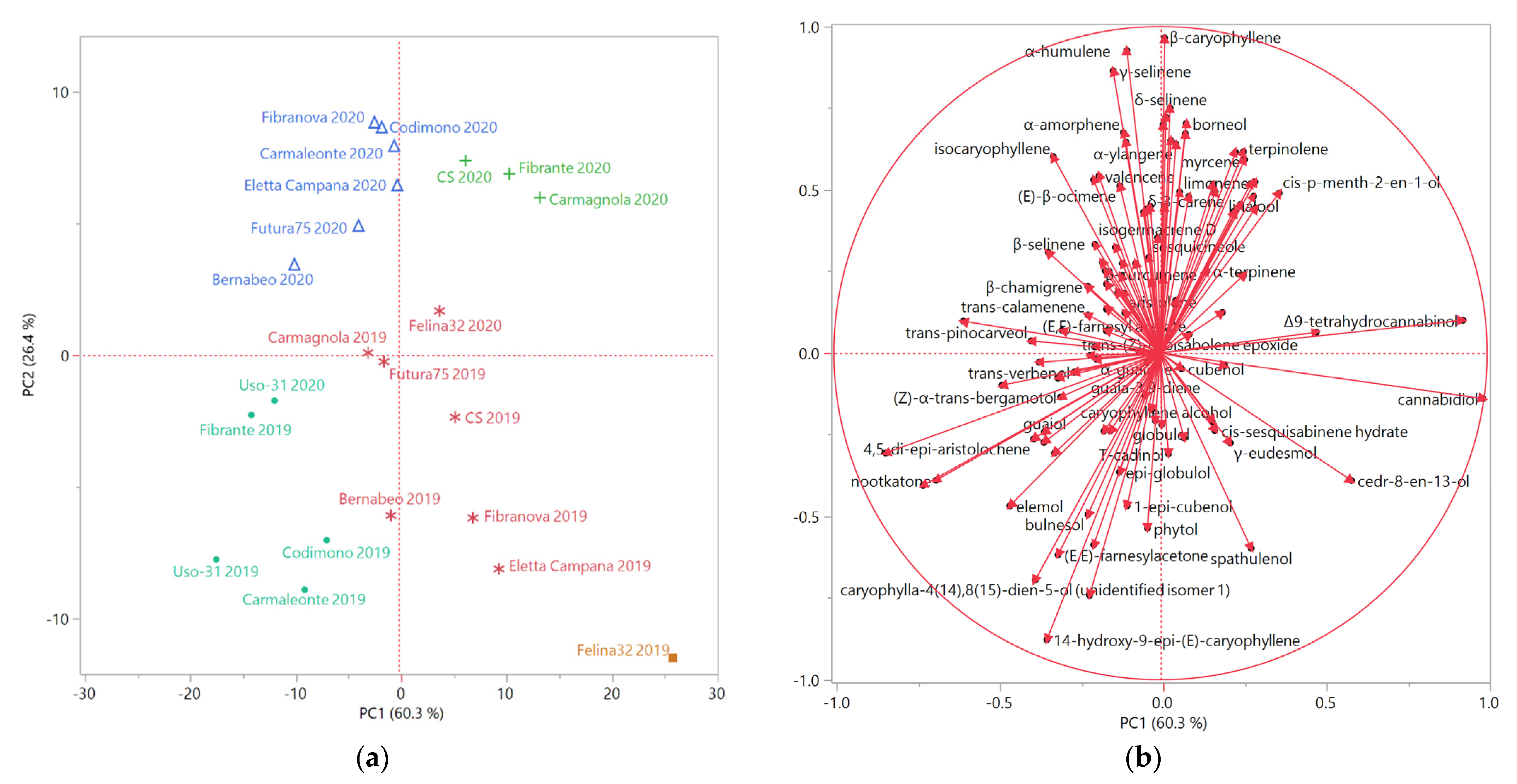
The score and loading plots obtained by the principal component analysis (PCA), performed on the complete chemical compositions of the EOs extracted from all the analyzed hemp samples, is reported in Figure 2a and 2b, respectively.
|
The distribution of the samples in the score plot evidenced a partition based on the year of cultivation, as the samples of 2019 were plotted in the bottom quadrants (PC2 < 0) of the score plot, and those of 2020 in the upper quadrants (PC2 > 0), with the exception of Uso-31-2020, which was placed in the bottom left quadrant (PC1 and PC2 < 0) as Uso-31-2019.
Furthermore, the grouping of the samples obtained by the PCA was comparable to that of the HCA. All the samples of the B macro-cluster, evidenced in light blue, were plotted in the bottom left quadrant (PC1 and PC2 < 0) of the PCA score plot, due to their relevant relative abundances in the oxygenated sesquiterpenes caryophyllene oxide, humulene oxide II, caryophylla-4(14),8(15)-dien-5-ol (isomer 1 or 2), and 14-hydroxy-9-epi-(E)-caryophyllene.
The samples of the A macro-cluster, instead, occupied the remaining three quadrants of the score plot, with few exceptions. In particular, the samples of the blue sub-cluster were plotted in the upper left quadrant (PC1 < 0 and PC2 > 0), while the EOs of the green cluster were positioned in the upper right quadrant (PC1 and PC2 > 0). The blue and the green sub-clusters included most of the 2020 EOs samples, excluding Uso-31-2020, as previously mentioned, and Felina 32-2020, belonging to the pink cluster, but also plotted in the upper right quadrant. The β-caryophyllene and α-humulene vectors were responsible for the positioning of the 2020 samples in the upper quadrants, whilst the discrimination between the left and the right quadrants was determined by minor sesquiterpenes hydrocarbons and monoterpenes vectors, respectively.
The only orange sub-cluster sample was plotted in the bottom right quadrant (PC1 > 0 and PC2 < 0), as three samples of the pink sub-group: this positioning was probably due to the cannabidiol vector. The other samples of the pink group were plotted very close to the bottom right quadrant: Bernabeo-2019 was plotted towards the rightmost area of the bottom left quadrant, due to its relevant relative abundances in cannabidiol, caryophyllene oxide and humulene oxide II. Although Bernabeo is reported as a CBG-chemotype cultivar, its presence in the EO was not detected: this was likely due to the lower volatility of CBG compared to CBD, its higher boiling point, and its overall higher polarity due to the positioning of its two hydroxyls on the benzenic ring, as they are less likely to engage in the formation of a pseudocyclic structure, as could, instead, occur in CBD. Finally, Futura 75-2019 and Carmagnola-2019, due to their relevant relative abundances in β-caryophyllene and α-humulene, were plotted next to each other, close to the PC1-PC2 axis intersection, on the left side of the score plot.
Materials and methods
Plant material
Plant material was obtained from experimental fields of CREA-CI located in Rovigo, Italy (45°04′43.4″ N 11°45′57.7″ E). For the two subsequent years of cultivation (2019–2020) the same fields were used, characterized by a cultural precession with straw cereals. Nitrogen fertilization was applied before sowing (40- and 54-units of nitrogen fertilizer in 2019 and 2020, respectively). Meteorological information (temperature and rainfall) was collected, and monthly averages are presented in Table 5.
The site had total rainfall during the outdoor growing season (from April to September) of 428 mm and 288 mm for 2019 and 2020, respectively. In the same period, 2019 had an average temperature of 21.06 °C, slightly higher than those registered for the same period in 2020, when the average temperature reached 20.48 °C. In both years, the maximum temperature never exceeded 32 °C in the hottest months. At harvest, each plant was cut at its lower third and air-dried at ambient temperature and in the dark, to avoid photo-oxidation reactions. Material for EOs analysis included inflorescences and floral bracts, which were separated manually from stems and seeds, with a 2 mm diameter sieve.
Essential oil hydrodistillation
The extraction of the essential oil from the plant material was performed by hydrodistillation with a standard Clevenger-type apparatus, for two hours. For both 2019 and 2020 samples, the hydrodistillation was carried out in triplicate, on 100 g of dried and shredded inflorescences and floral bracts, previously macerated in water under mechanical agitation (150 rpm) for 24 hours prior to hydrodistillation. The collected essential oils were stored in a refrigerator until analysis.
Gas chromatography—mass spectrometry analyses and peaks identification
The essential oils were diluted to 0.5% in high-performance liquid chromatography (HPLC)-grade n-hexane and then injected into a gas chromatography–mass spectrometry (GC–MS) apparatus. The gas chromatography–electron ionization mass spectrometry (GC–EIMS) analyses were performed with an Agilent 7890B gas chromatograph (Agilent Technologies Inc., Santa Clara, CA, USA) equipped with an Agilent HP-5MS capillary column (30 m × 0.25 mm; coating thickness 0.25 µm) and an Agilent 5977B single quadrupole mass detector. The analytical conditions were as follows: oven temperature programmed from 60 to 240 °C at 3 °C/min; injector temperature, 220 °C; transfer line temperature, 240 °C; carrier gas helium, 1 mL/min. The injection volume was 1 μL, with a split ratio of 1:25. The acquisition parameters were as follows: full scan; scan range: 30–300 m/z; scan time: 1.0 s.
The identification of the constituents was based on a comparison between the retention times with those of the authentic samples, comparing their linear retention indices relative to the series of n-hydrocarbons. Computer matching was also used against commercial (NIST 14 and ADAMS 2007) and laboratory-developed mass spectra libraries built up from pure substances and components of commercial essential oils of known composition and the MS literature data.[35][36][37][38][39][40]
Statistical analyses
The percentage of dissimilarity contribution of all the compounds of C. sativa EOs was evaluated by means of the Similarity Percentage Test (SIMPER) with the Bray–Curtis distance/similarity measure. The statistical significance of the difference in the relative abundances of the compounds accounting for at least 1.00% in the dissimilarity rate of the emissions was evaluated using the F- or t-test, for compounds with equal or unequal variances, respectively. The SIMPER, F- and t-tests were performed with the Past 4.03 Software.[41]
The analysis of variance (ANOVA) was carried out using the JMP Pro 14 software package (SAS Institute, Cary, NC, USA). A two-way ANOVA was carried out in order to estimate the variance components of genotypes (G; Bernabeo, Carmagnola, Carmaleonte, Codimono, CS, Eletta Campana, Felina 32, Fibranova, Fibrante, Futura 75, and Uso-31), year of cultivation (Y; 2019 and 2020), and their interaction (G × Y). Averages were separated by Tukey’s b post-hoc test. p < 0.05 was used to assess the significance of differences between means.
The multivariate statistical analyses were also carried out with the JMP software package. The covariance data matrix for the statistical evaluation of the EOs composition was a 116 × 22 matrix (116 compounds × 22 samples = 2.552 data). The principal component analysis (PCA) was performed selecting the two highest principal components (PCs) obtained by the linear regressions operated on mean-centered, unscaled data; as an unsupervised method, this analysis aimed at reducing the dimensionality of the multivariate data of the matrix, whilst preserving most of the variance. The principal component analysis (PCA) was performed, selecting the two highest PCs obtained by the linear regressions: the chosen PC1 and PC2 cover 60.30 and 26.40% of the variance, respectively, for a total explained variance of 86.70%. The hierarchical cluster analysis (HCA) was performed using Ward’s method, with squared Euclidean distances as a measure of similarity. Both the HCA and the PCA methods can be applied to observe groups of samples, even when there are no reference samples that can be used as a training set to establish the model.
Conclusions
The essential oils obtained from hemp inflorescences constituted a high-value derivative by-product, contributing to a more sustainable agricultural system. The present study aimed to evaluate the chemical composition and the yield of the EO obtained from 11 genotypes of hemp, cultivated and collected for two consecutive growing seasons in the same cultural conditions, to promote their employment based on their peculiar characteristics.
The results of the present study showed that sesquiterpenes and cannabinoids were the main classes of compounds of the hemp essential oils obtained from dried inflorescences. Regarding the sesquiterpenes, the hydrocarbon form was more abundant in 2020 samples, while the oxygenated one was predominant in 2019 EOs: the major compounds belonging to this chemical class were β-caryophyllene, α-humulene, their oxygenated derivatives caryophyllene oxide, 14-hydroxy-9-epi-(E)-caryophyllene, and humulene oxide II, all typical components of hemp EO. The cannabinoids identified in all the samples were cannabidiol, cannabichromene, and Δ9-tetrahydrocannabinol, but only the former was revealed in relevant percentages. The EO extraction yield ranged from 0.03 to 0.12% w/w in 2019 samples, whilst in the 2020 ones, it was comprised between 0.03 and 0.23% w/w. The obtained data have shown that both the EO chemical profile and extraction yield were significantly influenced by the genotype of the starting material, the year of cultivation, and the interaction between these two factors.
Acknowledgements
Author contributions
Conceptualization, R.A., G.F., L.P., L.B.; methodology, R.A., G.F., Y.P.; software R.A., Y.P.; validation, R.A., G.F., Y.P.; formal analysis, R.A., Y.P.; investigation, Y.P., V.T.; resources, G.F., L.P., R.P., L.B., F.F., M.M.; data curation, R.A., Y.P., F.F.; writing—original draft preparation, R.A., Y.P.; writing—review and editing, R.A., G.F., L.P., Y.P.; visualization, R.A., Y.P.; supervision, G.F. and L.P.; project administration, G.F., L.P., R.P.; funding acquisition, G.F., L.P., R.P., M.M. All authors have read and agreed to the published version of the manuscript.
Funding
The plant material used in this study was provided in the frame of the following projects: MAIDET research project “Metodi analitici innovativi per la determinazione del THC in matrici vegetali”, funded by the Ministero delle Politiche Agricole Alimentari e Forestali (MIPAAF), grant decree D.M.34176/7303/2017; UNIHEMP research project “Use of iNdustrIal Hemp biomass for Energy and new biocheMicals Production” (ARS01_00668) funded by European Regional Development Fund (within the PON R&I 2017-2020– Axis 2 – Action II – OS 1.b). Grant decree UNIHEMP prot. no. 2016 of 27/07/2018; CUP B76C18000520005; and PROCAFAA “PRODURRE CANAPA NELLA FILIERA ALIMENTARE E AGROINDUSTRIALE” PSR Veneto 2014-2020. Reg. (UE) n. 1305/2013, Art. 35. DGR no. 736 of 28 May 2018. Misura 16 “Cooperazione”. Measures 16.1.1 e 16.2.1. BURV n°38 of 19 April 2019.
Data availability
Data is contained within the article.
Sample availability
Samples of the compounds are available from the authors.
Conflicts of interest
The authors declare no conflict of interest.
References
- ↑ 1.0 1.1 Bonini, S.A.; Premoli, M.; Tambaro, S. et al. (2018). "Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history". Journal of Ethnopharmacology 227: 300–15. doi:10.1016/j.jep.2018.09.004.
- ↑ Chandra, S.; Lata, H.; Khan, I.A. et al. (2017). "Chapter 3: Cannabis sativa L.: Botany and Horticulture". In Chandra, S.; Lata, H.; ElSohly, M.A.. Cannabis sativa L. - Botany and Biotechnology. Springer. pp. 79–100. ISBN 9783319545646.
- ↑ Pollio, A. (2016). "The Name of Cannabis: A Short Guide for Nonbotanists". Cannabis and Cannabinoid Research 1 (1): 234–38. doi:10.1089/can.2016.0027. PMC PMC5531363. PMID 28861494. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC5531363.
- ↑ Flores-Sanchez, I.J.; Verpoorte, R. (2008). "Secondary metabolism in cannabis". Phytochemistry Reviews 7: 615–39. doi:10.1007/s11101-008-9094-4.
- ↑ Fernandez, E.B.; Peterseil, V.; Menges, S. et al. (2020). "Distribution of Chemical Phenotypes (Chemotypes) in European Agricultural Hemp (Cannabis sativa L.) Cultivars". Journal of Forensic Sciences 65 (3): 715–21. doi:10.1111/1556-4029.14242.
- ↑ Mandolino, G.; Carboni, A. (2004). "Potential of marker-assisted selection in hemp genetic improvement". Euphytica 140: 107–20. doi:10.1007/s10681-004-4759-6.
- ↑ European Commission (27 December 2020). "Commission Regulation (EC) No 2860/2000 of 27 December 2000 amending Regulation (EC) No 2316/1999 laying down detailed rules for the application of Council Regulation (EC) No 1251/1999 establishing a support system for producers of certain arable crops, to include flax and hemp grown for fibre, specifying the rules on set-aside areas and amending the base areas for Greece and Portugal". Publications Office of the European Union. https://op.europa.eu/en/publication-detail/-/publication/3700d4bc-0c60-4f10-a329-b41db5b3e57c/language-en.
- ↑ 8.0 8.1 8.2 8.3 Ascrizzi, R.; Ceccarini, L.; Tavarini, S. (2019). "Valorisation of hemp inflorescence after seed harvest: Cultivation site and harvest time influence agronomic characteristics and essential oil yield and composition". Industrial Crops and Products 139: 111541. doi:10.1016/j.indcrop.2019.111541.
- ↑ 9.0 9.1 Ascrizzi, R.; Iannone, M.; Cinque, G. et al. (2020). "“Hemping” the drinks: Aromatizing alcoholic beverages with a blend of Cannabis sativa L. flowers". Food Chemistry 325: 126909. doi:10.1016/j.foodchem.2020.126909.
- ↑ 10.0 10.1 10.2 Benelli, G.; Pavela, R.; Petrelli, R. et al. (2018). "The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops". Industrial Crops and Products 122: 308-315. doi:10.1016/j.indcrop.2018.05.032.
- ↑ Bedini, S.; Flamini, G.; Cosci, F. et al. (2016). "Cannabis sativa and Humulus lupulus essential oils as novel control tools against the invasive mosquito Aedes albopictus and fresh water snail Physella acuta". Industrial Crops and Products 85: 318-323. doi:10.1016/j.indcrop.2016.03.008.
- ↑ Agnieszka, S.; Magdalena, R.; Jan, B. et al. (2016). "Phytotoxic Effect of Fiber Hemp Essential Oil on Germination of Some Weeds and Crops". Journal of Essential Oil Bearing Plants 19 (2): 262–76. doi:10.1080/0972060X.2015.1137236.
- ↑ Orlando, G.; Adorisio, S.; Delfino, D. et al. (2021). "Comparative Investigation of Composition, Antifungal, and Anti-Inflammatory Effects of the Essential Oil from Three Industrial Hemp Varieties from Italian Cultivation". Antibiotics 10 (3): 334. doi:10.3390/antibiotics10030334.
- ↑ Mediavilla, V.; Steinemann, S. (1997). "Essential oil of Cannabis sativa L. strains". Journal of the International Hemp Association 4 (2): 80–2. http://druglibrary.net/olsen/HEMP/IHA/jiha4208.html.
- ↑ Fischedick, J.T. (2017). Identification of Terpenoid Chemotypes Among High (-)- trans-Δ 9- Tetrahydrocannabinol-Producing Cannabis sativa L. Cultivars. 2. pp. 34–47. doi:10.1089/can.2016.0040. PMC PMC5436332. PMID 28861503. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC5436332.
- ↑ Andre, C.M.; Hausman, J.-F.; Guerriero, G. (2016). "Cannabis sativa: The Plant of the Thousand and One Molecules". Frontiers in Plant Science 7: 19. doi:10.3389/fpls.2016.00019. PMC PMC4740396. PMID 26870049. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC4740396.
- ↑ Sommano, S.R.; Chittashupho, C.; Ruksiriwanich, W. et al. (2020). "The Cannabis Terpenes". Molecules 25 (24): 5792. doi:10.3390/molecules25245792. PMC PMC7763918. PMID 33302574. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC7763918.
- ↑ 18.0 18.1 18.2 18.3 Vuerich, M.; Ferfuia, C.; Zuliani, F. et al. (2019). "Yield and Quality of Essential Oils in Hemp Varieties in Different Environments". Agronomy 9 (7): 356. doi:10.3390/agronomy9070356.
- ↑ 19.0 19.1 19.2 Abdollahi, M.; Sefidkon, F.; Calagari, M. et al. (2020). "Impact of four hemp (Cannabis sativa L.) varieties and stage of plant growth on yield and composition of essential oils". Industrial Crops and Products 155: 112793. doi:10.1016/j.indcrop.2020.112793.
- ↑ Gilbert, A.N.; DiVerdi, J.A. (2018). "Consumer perceptions of strain differences in Cannabis aromas". PLoS ONE 13 (2): e0192247. doi:10.1371/journal.pone.0192247.
- ↑ 21.0 21.1 21.2 Bertoli, A.; Tozzi, S.; Pistelli, L. et al. (2010). "Fibre hemp inflorescences: From crop-residues to essential oil production". Industrial Crops and Products 32 (3): 329–37. doi:10.1016/j.indcrop.2010.05.012.
- ↑ 22.0 22.1 22.2 22.3 Meier, C.; Mediavilla, V. (1998). "Factors influencing the yield and the quality of hemp (Cannabis sativa L.) essential oil". Journal of the International Hemp Association 5 (1): 16–20. http://druglibrary.net/olsen/HEMP/IHA/jiha4208.html.
- ↑ Campiglia, E.; Radicetti, E.; Mancinelli, R. (2017). "Plant density and nitrogen fertilization affect agronomic performance of industrial hemp (Cannabis sativa L.) in Mediterranean environment". Industrial Crops and Products 100: 246–54. doi:10.1016/j.indcrop.2017.02.022.
- ↑ Tejero, I.F.G.; Zuazo, V.H.D.; Perez-Alvarez, R. et al. (2014). "Impact of Plant Density and Irrigation on Yield of Hemp (Cannabis sativa L.) in a Mediterranean Semi-arid Environment". Journal of Agricultural Science and Technology 16 (4): 887–95. http://jast.modares.ac.ir/article-23-9987-en.html.
- ↑ 25.0 25.1 Menghini, L; Ferrante, C.; Carradori, S. et al. (2021). "Chemical and Bioinformatics Analyses of the Anti-Leishmanial and Anti-Oxidant Activities of Hemp Essential Oil". Biomolecules 11 (2): 272. doi:10.3390/biom11020272. PMC PMC7917915. PMID 33673274. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC7917915.
- ↑ 26.0 26.1 Hanuš, L.O.; Hod, Y. (2020). "Terpenes/Terpenoids in Cannabis: Are They Important?". Medical Cannabis and Cannabinoids 3: 25–60. doi:10.1159/000509733.
- ↑ 27.0 27.1 27.2 27.3 Micalizzi, G.; Alibrando, F.; Vento, F. et al. (2021). "Development of a Novel Microwave Distillation Technique for the Isolation of Cannabis sativa L. Essential Oil and Gas Chromatography Analyses for the Comprehensive Characterization of Terpenes and Terpenoids, Including Their Enantio-Distribution". Molecules 26 (6): 1588. doi:10.3390/molecules26061588. PMC PMC8000122. PMID 33805665. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC8000122.
- ↑ 28.0 28.1 Nissen, L.; Zatta, A.; Stefanini, I. et al. (2010). "Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.)". Fitoterapia 81 (5): 413–9. doi:10.1016/j.fitote.2009.11.010. PMID 19969046.
- ↑ Wanas, A.S.; Radwan, M.M.; Chandra, S. (2020). "Chemical Composition of Volatile Oils of Fresh and Air-Dried Buds of Cannabis chemovars, Their Insecticidal and Repellent Activities". Natural Product Communications 15 (5): 1–7. doi:10.1177/1934578X20926729.
- ↑ 30.0 30.1 30.2 Fiorini, D.; Molle, A.; Nabissi, M. et al. (2019). "Valorizing industrial hemp (Cannabis sativa L.) by-products: Cannabidiol enrichment in the inflorescence essential oil optimizing sample pre-treatment prior to distillation". Industrial Crops and Products 128: 581–89. doi:10.1016/j.indcrop.2018.10.045.
- ↑ Eržen, M.; Košir, I.J.; Ocvirk, M. et al. (2021). "Metabolomic Analysis of Cannabinoid and Essential Oil Profiles in Different Hemp ( Cannabis sativa L.) Phenotypes". Plants 10 (5): 966. doi:10.3390/plants10050966. PMC PMC8151046. PMID 34066131. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC8151046.
- ↑ Hazekamp, A.; Fischedick, J.T. (2012). "Cannabis - from cultivar to chemovar". Drug Testing and Analysis 4 (7–8): 660–7. doi:10.1002/dta.407. PMID 22362625.
- ↑ Fischedick, J.T.; Hazekamp, A.; Erkelens, T. et al. (2010). "Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes". Phytochemistry 71 (17–18): 2058–73. doi:10.1016/j.phytochem.2010.10.001. PMID 21040939.
- ↑ Elzinga, S.; Fischedick, J.; Podkolinski, R. et al. (2015). "Cannabinoids and Terpenes as Chemotaxonomic Markers in Cannabis". Natural Products Chemistry & Research 3: 181. doi:10.4172/2329-6836.1000181.
- ↑ Adams, R.P. (2007). Identification of Essential Oil Components By Gas Chromatography/Mass Spectrometry. Allured Publishing Corp. ISBN 9781932633214.
- ↑ Davies, N.W. (1990). "Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases". Journal of Chromatography A 503: 1–24. doi:10.1016/S0021-9673(01)81487-4.
- ↑ Jennings, W.; Shibamoto, T. (1980). Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography. Academic Press. doi:10.1016/B978-0-12-384250-3.X5001-6. ISBN 9780123842503.
- ↑ Masada, Y. (1976). Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry. John Wiley & Sons. ISBN 047015019X.
- ↑ Stenhagen, E.; Abrahamsson, S.; McLafferty, F.W. (1974). Registry of Mass Spectral Data. John Wiley & Sons. ISBN 0471821152.
- ↑ Swigar, A.A.; Silverstein, R.M. (1981). Monoterpenes. Aldrich Chemical Company.
- ↑ Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. (2001). PAST: Paleontological Statistics Software Package for Education and Data Analysis. 4. pp. 1–9. https://palaeo-electronica.org/2001_1/past/issue1_01.htm.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added.