Difference between revisions of "Journal:Differentiating cannabis products: Drugs, food, and supplements"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 119: | Line 119: | ||
The pathways by which exogenous cannabinoids such as Δ<sup>9</sup>-THC and CBD interact with the human endocannabinoid system (ECS) are complex and only partially understood. Definitively, THC has been shown to directly bind CB1 and CB2 receptors, exerting psychoactive and other neurologically-mediated effects. CBD does not directly bind CB1 or CB2 receptors, exhibiting a low affinity for both, but can antagonize synthetic agonists of cannabinoid receptors, suggesting a negative allosteric modulatory effect. [Freeman et al., 2019; Premoli et al., 2019] Although CBD does not agonize CBR1 or CBR2, it exhibits several cannabinoid receptor-independent activities, including direct agonism of TRPV1 and 5-HT1A receptors, enhancement of adenosine receptor signaling, upregulation of peroxisome proliferator activated receptor gamma (PPARγ) protein expression, and suppression of GPR55 receptor activity. [Russo & Marcu 2017] CBD is non-intoxicating with a wide therapeutic index and acceptable side effect profile, and has pre-clinically exerted antipsychotic, intestinal anti-prokinetic, neuroprotective, anti-proliferative, anti-ischemic, vasorelaxant, analgesic, anxiolytic, anti-inflammatory, and antiepileptic effects. [Noreen et al., 2018; Cerino et al., 2021] CBD also has been shown to negate the unpleasant side effects of THC—including anxiety, psychosis, tachycardia, and drowsiness—through negative allosteric modulation of CB1 receptors. [Laprairie et al., 2015] | The pathways by which exogenous cannabinoids such as Δ<sup>9</sup>-THC and CBD interact with the human endocannabinoid system (ECS) are complex and only partially understood. Definitively, THC has been shown to directly bind CB1 and CB2 receptors, exerting psychoactive and other neurologically-mediated effects. CBD does not directly bind CB1 or CB2 receptors, exhibiting a low affinity for both, but can antagonize synthetic agonists of cannabinoid receptors, suggesting a negative allosteric modulatory effect. [Freeman et al., 2019; Premoli et al., 2019] Although CBD does not agonize CBR1 or CBR2, it exhibits several cannabinoid receptor-independent activities, including direct agonism of TRPV1 and 5-HT1A receptors, enhancement of adenosine receptor signaling, upregulation of peroxisome proliferator activated receptor gamma (PPARγ) protein expression, and suppression of GPR55 receptor activity. [Russo & Marcu 2017] CBD is non-intoxicating with a wide therapeutic index and acceptable side effect profile, and has pre-clinically exerted antipsychotic, intestinal anti-prokinetic, neuroprotective, anti-proliferative, anti-ischemic, vasorelaxant, analgesic, anxiolytic, anti-inflammatory, and antiepileptic effects. [Noreen et al., 2018; Cerino et al., 2021] CBD also has been shown to negate the unpleasant side effects of THC—including anxiety, psychosis, tachycardia, and drowsiness—through negative allosteric modulation of CB1 receptors. [Laprairie et al., 2015] | ||
Other prominent cannabaninoids of pharmaceutical interest include CBG, CBC, CBN, [[tetrahydrocannabivarin]] (THCV), [[cannabidivarin]] (CBDV), and the acidic cannabinoid precursors THCA and CBDA. Most of these cannabinoids are still being investigated in preliminary ''in vitro'' and animal model research studies; however, some mechanisms of action have been elucidated and reported in the literature. CBG, for example, has weak affinity for CB1 and CB2 receptors, but is a potent α2-adrenoreceptor agonist, an efficient serotonin 5-HT1A receptor antagonist, and might activate a number of other dominant receptors involved in heat/cold sensitization, pain, and inflammation via antagonism of TRPV8 receptors and stimulation of TRPV1, TRPV2, TRPA1, TRPV3, and TRPV4. [Cascio et al., 2010; De Petrocellis & Di Marzo, 2010; De Petrocellis et al., 2011; Morales et al., 2017] CBC can have profound therapeutic effects on inflammation and pain through CB2 receptor activity and stimulation and desensitization of TRP ankyrin-type 1 (TRPA1) cation channels, interactions with TRPV3 and TRPV4 cation channels, and desensitization of TRPV2 and TRPV4 channels. [De Petrocellis et al., 2012; Cascio & Pertwee, 2014] CBC has also been shown to relieve pain in mice by augmenting the analgesic effects of THC when the two are co-administered [Davis & Hatoum, 1983; Maione et al., 2011; Cascio & Pertwee, 2014], and it can increase concentrations and prolong the duration of endocannabinoids like anandamide through its interactions with TRP channels. [Shinjyo & Di Marzo, 2013; De Petrocellis et al., 2011, 2012] CBN is being investigated for several topical therapeutic targets for skin conditions like psoriasis, burns, and MRSA infections, via inhibition of keratinocyte proliferation, TRPV2 agonism, and direct antimicrobial activity, respectively. [Wilkinson & Williamson, 2007; Appendio et al., 2008; Qin et al., 2008; Russo, 2014] Additionally, CBN is an important marker compound of THC degradation in plant material, an artifact that occurs naturally with storage and may be expedited by high heat exposure as the oxidation byproduct of THC. [Upton et al., 2013] | |||
THCV, a propyl analogue of THC, exhibits concentration-dependent activity at CB1 receptors and may perform as an agonist or act as an antagonist, accordingly. [Pertwee, 2008] THCV demonstrates anticonvulsant attributes in mouse pyriform cortex and cerebellum [Hill et al., 2010], and it may promote weight loss and other cardiometabolic benefits. [Riedel et al., 2009] CBDV has been reported to have significant anticonvulsant activity and is potentially of equal therapeutic value to CBD in treating epilepsy, especially focal/partial onset seizures. [Williams, Jones, & Whalley, 2014] CBDV also activates or blocks a wide range of cation channels, depending on the concentration and experimental conditions, including TRPA1, TRPM8, and TRPV channels 1–4. Of additional importance, CBDV inhibits the cellular uptake of anandamide and inhibits endocannabinoid degradation through modulating enzymes diacylglycerol lipase and N-acylethanolamine-hydrolyzing acid amidase (NAAA). [Pertwee & Cascio, 2014] | |||
THCA is the acidic precursor of THC, representing up to 90% of total THC in the plant prior to prolonged storage, UV exposure, or heat. [Moreno- Sanz, 2016] Like THC, THCA is an agonist of CB1 and CB2 receptors, but there is discrepancy as to which (THC or THCA) has greater affinity. [Verhoeckx et al., 2006; Rock et al., 2013; Rosenthaler et al., 2014] Although several cannabinoids have been shown to deposit in brain tissue [Alozie, et al., 1980; Deiana et al., 2012], THCA seems to have limited access to the CNS or ability to cross the blood brain barrier (BBB), likely due to its carboxylic acid group. [Moreno-Sanz et al., 2016] This means it also does not elicit any psychoactivity. Preliminary research suggests THCA exhibits antineoplastic, neuroprotective, and anti-inflammatory activities via modulation of COX pathways, TRP cation channels, and other immunomodulatory and cell signaling pathways. CBDA, the acidic precursor to CBD, shares the same enhancing activity of CBD at 5-HT1A receptors, even up to 10 times higher than the activity of CBD, but does not show agonism or antagonism at CB1 receptors. [Bolognini et al., 2013; McPartland et al., 2015] CBDA can also inhibit COX1 and COX2, and, at low concentrations (between 1 and 10 μM), targets GPR55, TRPA1, TRPV1, and TRPM8. [Takeda et al., 2008] | |||
Revision as of 18:03, 7 January 2023
| Full article title | Differentiating cannabis products: Drugs, food, and supplements |
|---|---|
| Journal | Frontiers in Pharmacology |
| Author(s) | Salehi, Arash; Puchalski, Keely; Shokoohinia, Yalda; Zolfaghari, Behzad; Asgary, Sedigheh |
| Author affiliation(s) | Isfahan University of Medical Sciences, Southwest College of Naturopathic Medicine |
| Primary contact | Email: sedighehasgary at gmail dot com |
| Editors | Lupica, Carl R. |
| Year published | 2022 |
| Volume and issue | 13 |
| Article # | 906038 |
| DOI | 10.3389/fphar.2022.906038 |
| ISSN | 1663-9812 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.frontiersin.org/articles/10.3389/fphar.2022.906038/full |
| Download | https://www.frontiersin.org/articles/10.3389/fphar.2022.906038/pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
"Hemp" refers to non-intoxicating, low delta-9 tetrahydrocannabinol (Δ9-THC) cultivars of Cannabis sativa L. "Marijuana" refers to cultivars with high levels of Δ9-THC, the primary psychoactive cannabinoid found in the plant and a federally controlled substance used for both recreational and therapeutic purposes. Although marijuana and hemp belong to the same genus and species, they differ in terms of chemical and genetic composition, production practices, product uses, and regulatory status. Hemp seed and hemp seed oil have been shown to have valuable nutritional capacity. Cannabidiol (CBD), a non-intoxicating phytocannabinoid with a wide therapeutic index and acceptable side effect profile, has demonstrated high medicinal potential in some conditions. Several countries and states have facilitated the use of THC-dominant medical cannabis for certain conditions, while other countries continue to ban all forms of cannabis regardless of cannabinoid profile or low psychoactive potential.
Today, differentiating between hemp and marijuana in the laboratory is no longer a difficult process. Certain thin-layer chromatography (TLC) methods can rapidly screen for cannabinoids, and several gas and liquid chromatography techniques have been developed for precise quantification of phytocannabinoids in plant extracts and biological samples. Geographic regulations and testing guidelines for cannabis continue to evolve. As they are improved and clarified, we can better employ the appropriate applications of this uniquely versatile plant from an informed scientific perspective.
Keywords: cannabis, cannabidiol, CBD, hemp, marijuana, tetrahydrocannabinol, THC
Introduction
Cannabis sativa L. is a member of the Cannabaceae family, a small family of annual herbaceous plants that includes the widely cultivated genus, Humulus (hops), and eight other genera. [Jin et al., 2019] The terms "hemp" and "marijuana" both refer to plants derived from the Cannabis sativa L. species, but from different cultivars or chemotypes having either low or high delta-9 tetrahydrocannabinol (Δ9-THC) content, respectively. The general term "cannabis" includes both hemp and marijuana types of plants. [Johnson, 2019] Cannabis has been utilized by several populations for millennia. Central and southeast Asia are considered plausible origins. [Steven et al., 2016] It has been claimed that the application of the herb for various purposes dates to 12,000 years ago based on Neolithic evidence found in Taiwan. [Li, 1974] Hemp is reputed to be the oldest cultivated fiber plant [Cherney and Small, 2016], and hemp seed and seed oil have historically been used as food. [Farinon et al., 2020] Marijuana has long been utilized for recreational as well as medical purposes. [Piluzza et al., 2013] The oldest report of the medical use of cannabis dates to 5,000 years ago when it was used for the treatment of fatigue, rheumatism, and malaria. [Abel, 2013] The Assyrians, Egyptians, Indians, Persians, Greeks, and Romans are all reported to have used cannabis for medical purposes. [Leghissa et al., 2018a]
Classifications for different cannabis species continues to be a highly debated topic amongst taxonomists and botanists. [Sawler 2015] Although some experts recognize three different species of Cannabis—C. sativa, C. indica, and C. ruderalis (Pollio, 2016)—and other experts only one monospecific species (C. sativa L.) with two subspecies (subsp. sativa and subsp. indica [Lam.]) [Small and Cronquist, 1976, UNODC.org], neither of these nomenclature systems accurately reflects the diversity and complexity of modern cannabis plants. Because cannabis species have been cultivated globally over many years to exhibit nearly indistinguishable phenotypes with overlapping genotypes, Cannabis sativa L. varieties are now most accurately identified by cultivar or chemotype, including specific cannabinoid profiling, and even subtyping. [Sarma et al., 2020] (Recently proposed suggestions for chemotyping will be discussed later in this article.)
Although it has valuable nutritional capacity, hemp material was initially cultivated mainly to produce textiles and ropes. Due to expanded applications of synthetic fibers over the past two centuries, its cultivation decreased precipitously. In recent years, hemp regained popularity due to the rediscovery of its nutritional benefits, economic value, and variety of medicinal uses. [Farinon et al., 2020] Despite the resurgence of hemp, ongoing efforts to curtail recreational drug use have prompted several countries and states to restrict the cultivation of cannabis varieties with high concentrations of Δ9-THC, the primary psychoactive metabolite of the plant. [Aguilar et al., 2018]
For the past several decades, a lack of understanding and proper differentiation between hemp- and marijuana-type plants has slowed the development of cannabis research on the potential health benefits of the plant. [Farinon et al., 2020] And despite having clearer definitions, improper use of these terms is still regularly seen in the literature. It is pertinent to clarify terminology and provide useful tools and legal definitions to differentiate between food, drug, and supplement derivatives of cannabis.
In this review we focus mainly on the question, “What is essential for defining Cannabis as a food, supplement, or drug?” We also investigate the nutritional potential of hempseed and seed oil, the medicinal benefits of certain phytocannabinoids (one of the predominant phytochemical groups in Cannabis) as drugs and supplements, and the regulatory status of cannabis and cannabis products across the globe. Finally, we review several analytical techniques for the detection and quantification of cannabinoids in cannabis extracts, samples, and other products.
Definitions, production practices, and uses
Cannabis terminology has been a significant source of confusion for many. In public opinion at large, marijuana is often viewed as a plant purposed solely for recreational use, which has overshadowed the high value of medical cannabis, hemp-type plants, and hemp products in nutrition, the health and wellness industry, scientific and medical communities, and the global economy. [Cadena, 2018] Because of this, it is increasingly important to implement clear literature-based nomenclature, legal definitions, and recommendations of agencies specializing in this controversial plant. Here we explore some of the current definitions and nomenclature.
Fundamentally, "marijuana" refers to cultivars of cannabis with psychoactive potential used for both recreational and therapeutic purposes, and "hemp" refers to non-intoxicating cultivars of cannabis with various end uses, including in fabrics and textiles, nutraceuticals, pharmaceuticals, food products, beverages, oral and topical self-care products, veterinary products, and other manufactured and industrial goods. The term "industrial hemp," in some cases, may be used interchangeably with "hemp." [Johnson, 2019; Crini et al., 2020]
With the passage of the Agriculture Improvement Act of 2018 (i.e., the 2018 Farm bill), hemp is now legally defined as "the plant species Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a total delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis." [1] Meanwhile, the Controlled Substances Act of 1970 (CSA) still broadly describes marijuana without using any limits on Δ9-THC or any other cannabinoid, defined as "all parts of the plant Cannabis sativa L., whether growing or not; the seeds thereof; the resin extracted from any part of such plant; and every compound, manufacture, salt, derivative, mixture, or preparation of such plant, its seeds, or resin." [2] Noticeably, this definition of marijuana excludes the mature stalks of Cannabis sativa L. and products derived from the stalks (e.g., fiber), as well as sterilized seeds incapable of germination, and any certain preparations of viable seeds, such as oil or cake, provided those preparations do not contain resin.
The enactment of the 2018 Farm bill effectively removed hemp from the legal definition of marijuana originally set forth in the CSA in 1970. The CSA had classified cannabis, including hemp, marijuana, and all associated cannabinoids, as a Schedule I controlled substance. The removal of hemp from controlled substance status allowed for agricultural cultivation of hemp plants on U.S. soil as well as greater federal oversight of hemp in commerce, including commodities like food and supplements. Although the separation of hemp from marijuana allowed significantly more freedom for hemp growers and product manufacturers—as well as researchers, clinicians, and consumers—the change also led to some new aspects of confusion, specifically surrounding use of the isolated compound cannabidiol (CBD). (We will later explore the nuances of CBD applications in food, pharmaceuticals, and supplements.)
The terms "hemp" and "marijuana" are both affiliated with lengthy cultural and political histories, and in the case of marijuana, polarizing ones. Today, these terms are no longer fully adequate to describe the numerous varieties and phytochemical complexities found in modern day cannabis, nor accurately describe their potential applications. In attempts to improve existing cannabis nomenclature, the United States Pharmacopeia (USP) Cannabis Expert Panel (CEP) suggested in 2020 that due to cannabis’s complex secondary metabolome and highly variable distribution of chemical constituents, three broad categories should be adopted for classifying cannabis based on phytocannabinoid chemotype: 1) THC-dominant chemotype; 2) intermediate chemotype with both THC and CBD; and 3) CBD-dominant chemotype. [Sarma et al., 2020] They also proposed that these categories could be further subcategorized by other cannabinoids and/or mono- and sesquiterpene profiles as we continue to learn more about the therapeutic potential of these other compounds in humans. [Sarma et al., 2020]
Hemp and marijuana plants generally share a common genetic trait pool with some predictable functional genetic variations; however, distinct genetic variations have also been observed amongst cannabis cultivars (particularly hemp cultivars) spanning the entire genome, not just genes related to THC or cannabinoid synthesis. [Sawler et al., 2015] Single nucleotide variant analysis demonstrates separation between THC and CBD. [Van Bakel et al., 2011] In most cases, the quantitative ratio of the phytochemicals tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA) (THCA:CBDA) may effectively be used for differentiating cannabis cultivars as either THC- or CBD-dominant (or intermediate), as THCA:CBDA ratios strongly reflect underlying genetic nuances in cannabinoid synthase enzymes (THCA and CBDA synthases) and their sequencing, loci distribution, patterns of expression (functional vs. non-functional), and affinities for cannabigerolic acid (CBGA), their shared substrate. [Weiblen et al., 2015] Weiblen et al. [2015] also proposed that all three types of cannabis chemotypes (THC-dominant, CBD-dominant, or intermediate) likely contain multiple linked loci with both THCA and CBDA synthase enzymes; however, identifying the presence of a non-functional CBDA synthase may directly indicate THC-dominance in a plant, as this non-functional enzyme has been selected for over many years where marijuana cultivation has thrived. This novel finding, if proven reliable as an identification method, would allow for rapid screening of THC- vs. CBD-dominant plant material at the genetic level prior to any plant cultivation.
Based on the genetic biosynthetic pathways of THC and CBD, Fernandez et al. [3] were able to report a systematic genetic prediction of Cannabis chemotypes of 62 European agricultural hemp cultivars. They suggested three main chemotype groupings for Cannabis sativa L., similar to the chemotypes proposed by the USP CEP[4]:
- Chemotype I, or THC-dominant varieties, in which the ratio of CBD/THC is low (0.00–0.05) and characterized by a THC level >0.3% (in the U.S.; dry weight of reproductive parts in the female plant at flowering time)
- Chemotype II, or THC-intermediate types, which have both THC and CBD in a content ratio (CBD/THC) of about 0.5–3.0
- Chemotype III, or CBD-predominant varieties, in which the ratio of CBD/THC is 15–25 and, in the U.S., contains <0.3% THC concentration (dry weight of reproductive part in female plants at flowering time)
These novel nomenclature systems eliminate much of the confusion surrounding hemp and marijuana, and they provide a destigmatized, more objective way of discussing Cannabis for certain applications. These classifications are particularly helpful when discussing the female inflorescence material of Cannabis plants, which is cultivated for many different chemotypes and cannabinoid concentrations (both THC- and CBD-dominant plants) for therapeutic use. For clarity, we will continue to use the three USP categories suggested above (THC-dominant, THC-intermediate, and CBD-dominant), where applicable, for the rest of this article.
In addition to the many chemotypes of plant material, there are also differences in production practices applied to cannabis. Based on different applications, hemp is generally cultivated for three different crops: fiber, seed, and CBD-dominant inflorescence. In contrast, THC-dominant plants are grown solely for their inflorescence for both medicinal and recreational use, as the inflorescence contains the most phytocannabinoids (i.e., Δ9-THC). [Johnson, 2019] Generally, all phytocannabinoids in cannabis exist in resin from the secretory glandular trichomes on the flowering tops of female plants. The numbers of these trichomes are fewer in male plants. [Hill et al., 2012] For this reason, female flowers are more valuable for phytocannabinoid production, while male plants are more appropriate for fiber production. Cannabis growers cultivating female plants for their phytocannabinoid production (generally CBD-, THC-, or CBD/THC co-dominant chemotypes) usually remove all male plants to prevent pollinating and seed yielding. Conversely, only male plants are grown to produce hemp fiber and seeds. Some hemp farmers producing fiber and seeds try to prevent flowering to encourage taller stalk growth and less branching [Johnson, 2019], whereas those cultivating hemp for CBD will promote growth of the plant’s phytocannabinoid-rich flowering tops.
Historically, there were significant visual differences between hemp and marijuana plants. Modern cultivated plants appear more phenotypically similar when grown for cannabinoid production, but some distinctions can still be made. Cultivated hemp for fiber and seed is usually characterized by a single tall stalk with few leaves and branches due to high-density cultivation in order to prevent branching, measuring up to 4.5 meters tall. In contrast, the flowering, phytocannabinoid-rich Cannabis plants (i.e., THC-dominant, THC-intermediate, or CBD-dominant chemotypes) are usually characterized by short, tightly clustered, bushy, low-density cultivation with many leaves and branches (in order to develop buds and flowers), and are only 1.2 meters tall. [Johnson, 2019]
Chemical and genetic composition
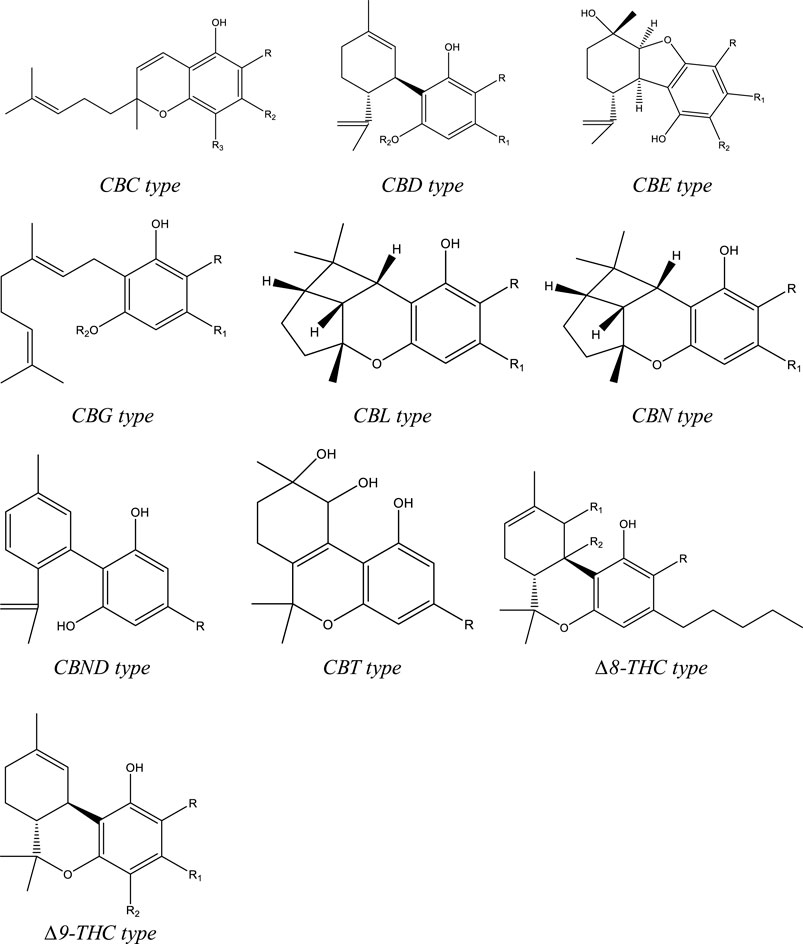
To date, more than 500 compounds have been identified from Cannabis sativa L., out of which 125 compounds are phytocannabinoids. Structurally these compounds have a C-21 terpenophenolic backbone consisting of an alkyl resorcinol with a monoterpene moiety. Eleven types are described for these cannabinoids based on their structure: Δ9-THC, CBD, (−)-Δ8-trans-tetrahydrocannabinol (Δ8-THC), cannabigerol (CBG), cannabinol (CBN), cannabichromene (CBC), cannabielsoin (CBE), cannabicyclol (CBL), cannabinodiol (CBND), cannabitriol (CBT), and miscellaneous-type cannabinoids (Figure 1). Among the phytocannabinoids, 25 of them are reported to be classified as Δ9-THC types and 10 have been elucidated as CBD types. [Hanus et al., 2016; Radwan et al., 2021] Apart from the cannabinoids, four major categories of non-cannabinoid-type compounds are present, including alkaloids, flavonoids, non-cannabinoid phenols, and terpenes. [Radwan 2021]
|
Although marijuana is technically defined as having greater than just 0.3% Δ9-THC concentration by dry weight in the U.S., today these plants are often bred to have Δ9-THC levels averaging as high as 10–30%. [Crini et al., 2020] Modern hemp plants cultivated for CBD production range anywhere from 5–15% total CBD. [Stack G.M. 2021] The number of cannabis plants cultivated for high CBD:THC ratios has grown exponentially in the past decade. Some researchers are finding that specific CBD-dominant chemovars have a CBDA synthase (CBDAS) gene that synthesizes not only CBDA, the precursor of CBD, but also synthesizes some THCA as a side product. [Stack G.M. 2021] With accumulation of CBD, where CBDAS is present, there may be concomitant accumulation of THC that exceeds most legal thresholds prior to the time that the plant accumulates the desired 10% or more total CBD. [Stack G.M. 2021] This illustrates the importance of chemovar selection and genetic testing prior to large scale cultivation.
Phytocannabinoids are the most abundant constituents in cannabis. Isoprenoids, also called terpenoids, are the second most abundant constituents, numbering over 200 total terpenes. [Russo 2011; Radwan 2021] Typically found in cannabis trichromes at about 10%, and in flowers at about 1%, some chemotypes have been bred to contain as much as 3.5% terpene content or higher in the flowers. [Potter, 2009; Fischedick et al., 2010] Although relatively modest in concentration to the cannabinoids, terpenes (mainly mono- and sesquiterpenes) are another important class of compounds. Terpenes are of increasing interest to researchers, healthcare practitioners, and the natural products industry as both isolated therapeutic agents and as enhancing compounds in holistic cannabis formulas, designed to engage what has recently been referred to as the entourage effect. [Sommano et al., 2020] Variation in terpene content is likely responsible for the more sedative vs. stimulating effects of different cannabis cultivars, a notion that had historically been attributed to indica vs. sativa varieties.
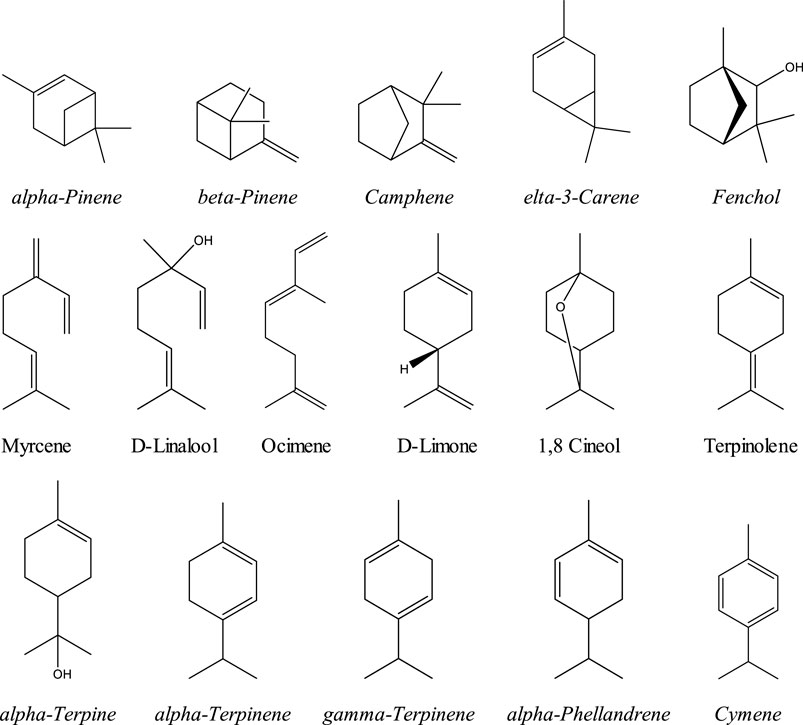
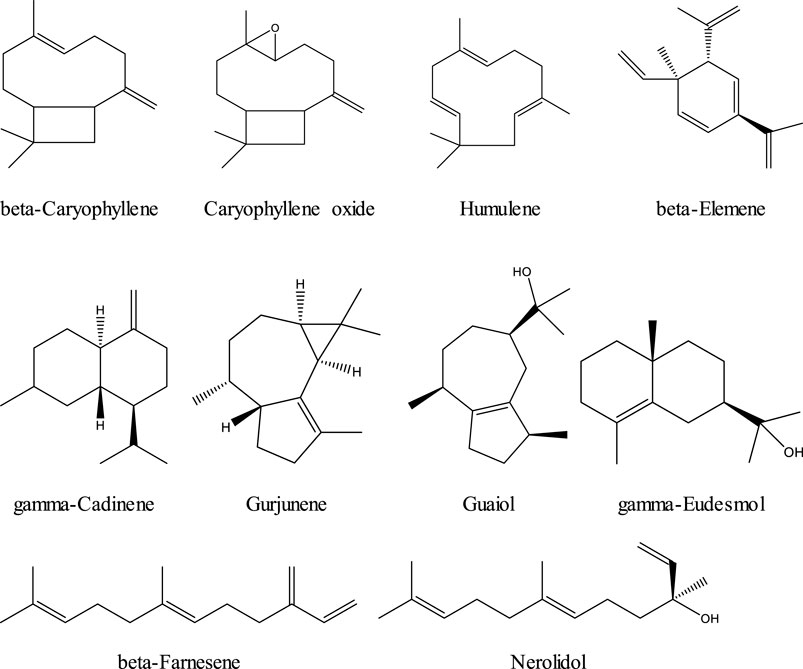
Terpenes are a family of organic compounds which are biosynthetically produced by isoprene units found mainly in plants. Certain cannabis terpenes have been identified and studied in plants such as tea tree, lavender, thyme, basil, Pinus sp., frankincense, and citrus fruits like lemon and mandarin. [Russo & Marcu 2017; Del Prado-Audelo ML et al., 2021] Monoterpenes and sesquiterpenes of cannabis (the volatile compounds) mainly exist in the essential oil, which is isolated by hydrodistillation. [Radwan et al., 2021] Among terpenes, the monoterpene β-myrcene is believed to have the highest prevalence in modern cannabis chemovars in the U.S. [Russo & Marcu 2017] The sixteen commonly encountered monoterpenes from three classes (acyclic, monocyclic, and bicyclic monoterpenes) are shown in Figure 2. The most common terpene that exists in cannabis extracts is the sesquiterpene β-caryophyllene. Sesquiterpenes have a 15-carbon skeleton. The most abundant sesquiterpenes of cannabis are shown in Figure 3. [Russo & Marcu 2017] However, other sources cite additional terpenes as most predominant based on samples from more specific geographic locations. [Fischedick, 2017; Radwan 2021]
|
|
Pharmacological effects
Cannabis is known to affect nearly every system in the human body, including but not limited to the central and peripheral nervous systems, as well as the endocrine, immune, gastrointestinal, and musculoskeletal systems. Thousands of articles and over 100 clinical studies have been published on the pharmacodynamics and bioactive effects of cannabis on human psychology, appetite, cognition, sleep, and pain. [Russo & Marcu 2017] Only in this century are we are beginning to understand the complexities of these pharmacological actions and interactions, which are largely due to the actions of cannabis on the human endocannabinoid system (ECS) and the “entourage effect” or bioactive synergisms between phytocannabinoids and other compounds within the plant.
The largest body of cannabis research prior to the past two or three decades focused primarily on the Δ9-THC as a medicinal and recreational drug and emphasized its psychoactive attributes and safety profile. In recent years, CBD has garnered more substantial research interest as a non-intoxicating but equally potent therapeutic compound. Structurally, Δ9-THC was elucidated in 1964 as the main psychoactive compound in cannabis. In 1988, Huestis et al. discovered that THC exerted its psychoactive effects through agonizing cannabinoid receptor 1 (CBR1). CBR1 is a G protein coupled receptor (GPCR) distributed widely throughout the central nervous system (CNS). A few years later, Murano et al. identified a second cannabinoid receptor, CBR2, that also exists in the CNS (in lower concentrations than CB1R), but in greater concentrations in the peripheral nervous system (PNS) and immune system. Two endogenous ligands (endocannabinoids) were later discovered to bind to these cannabinoid receptors: N-arachidonoyl ethanolamide (AEA) and 2-arachidonoyl glycerol (2-AG). [Lader et al., 2009] AEA and 2-AG are physiologic lipid-based retrograde neurotransmitters that interact with the ECS receptors and proteins in a similar manner to exogenous phytocannabinoids THC and CBD.
The pathways by which exogenous cannabinoids such as Δ9-THC and CBD interact with the human endocannabinoid system (ECS) are complex and only partially understood. Definitively, THC has been shown to directly bind CB1 and CB2 receptors, exerting psychoactive and other neurologically-mediated effects. CBD does not directly bind CB1 or CB2 receptors, exhibiting a low affinity for both, but can antagonize synthetic agonists of cannabinoid receptors, suggesting a negative allosteric modulatory effect. [Freeman et al., 2019; Premoli et al., 2019] Although CBD does not agonize CBR1 or CBR2, it exhibits several cannabinoid receptor-independent activities, including direct agonism of TRPV1 and 5-HT1A receptors, enhancement of adenosine receptor signaling, upregulation of peroxisome proliferator activated receptor gamma (PPARγ) protein expression, and suppression of GPR55 receptor activity. [Russo & Marcu 2017] CBD is non-intoxicating with a wide therapeutic index and acceptable side effect profile, and has pre-clinically exerted antipsychotic, intestinal anti-prokinetic, neuroprotective, anti-proliferative, anti-ischemic, vasorelaxant, analgesic, anxiolytic, anti-inflammatory, and antiepileptic effects. [Noreen et al., 2018; Cerino et al., 2021] CBD also has been shown to negate the unpleasant side effects of THC—including anxiety, psychosis, tachycardia, and drowsiness—through negative allosteric modulation of CB1 receptors. [Laprairie et al., 2015]
Other prominent cannabaninoids of pharmaceutical interest include CBG, CBC, CBN, tetrahydrocannabivarin (THCV), cannabidivarin (CBDV), and the acidic cannabinoid precursors THCA and CBDA. Most of these cannabinoids are still being investigated in preliminary in vitro and animal model research studies; however, some mechanisms of action have been elucidated and reported in the literature. CBG, for example, has weak affinity for CB1 and CB2 receptors, but is a potent α2-adrenoreceptor agonist, an efficient serotonin 5-HT1A receptor antagonist, and might activate a number of other dominant receptors involved in heat/cold sensitization, pain, and inflammation via antagonism of TRPV8 receptors and stimulation of TRPV1, TRPV2, TRPA1, TRPV3, and TRPV4. [Cascio et al., 2010; De Petrocellis & Di Marzo, 2010; De Petrocellis et al., 2011; Morales et al., 2017] CBC can have profound therapeutic effects on inflammation and pain through CB2 receptor activity and stimulation and desensitization of TRP ankyrin-type 1 (TRPA1) cation channels, interactions with TRPV3 and TRPV4 cation channels, and desensitization of TRPV2 and TRPV4 channels. [De Petrocellis et al., 2012; Cascio & Pertwee, 2014] CBC has also been shown to relieve pain in mice by augmenting the analgesic effects of THC when the two are co-administered [Davis & Hatoum, 1983; Maione et al., 2011; Cascio & Pertwee, 2014], and it can increase concentrations and prolong the duration of endocannabinoids like anandamide through its interactions with TRP channels. [Shinjyo & Di Marzo, 2013; De Petrocellis et al., 2011, 2012] CBN is being investigated for several topical therapeutic targets for skin conditions like psoriasis, burns, and MRSA infections, via inhibition of keratinocyte proliferation, TRPV2 agonism, and direct antimicrobial activity, respectively. [Wilkinson & Williamson, 2007; Appendio et al., 2008; Qin et al., 2008; Russo, 2014] Additionally, CBN is an important marker compound of THC degradation in plant material, an artifact that occurs naturally with storage and may be expedited by high heat exposure as the oxidation byproduct of THC. [Upton et al., 2013]
THCV, a propyl analogue of THC, exhibits concentration-dependent activity at CB1 receptors and may perform as an agonist or act as an antagonist, accordingly. [Pertwee, 2008] THCV demonstrates anticonvulsant attributes in mouse pyriform cortex and cerebellum [Hill et al., 2010], and it may promote weight loss and other cardiometabolic benefits. [Riedel et al., 2009] CBDV has been reported to have significant anticonvulsant activity and is potentially of equal therapeutic value to CBD in treating epilepsy, especially focal/partial onset seizures. [Williams, Jones, & Whalley, 2014] CBDV also activates or blocks a wide range of cation channels, depending on the concentration and experimental conditions, including TRPA1, TRPM8, and TRPV channels 1–4. Of additional importance, CBDV inhibits the cellular uptake of anandamide and inhibits endocannabinoid degradation through modulating enzymes diacylglycerol lipase and N-acylethanolamine-hydrolyzing acid amidase (NAAA). [Pertwee & Cascio, 2014]
THCA is the acidic precursor of THC, representing up to 90% of total THC in the plant prior to prolonged storage, UV exposure, or heat. [Moreno- Sanz, 2016] Like THC, THCA is an agonist of CB1 and CB2 receptors, but there is discrepancy as to which (THC or THCA) has greater affinity. [Verhoeckx et al., 2006; Rock et al., 2013; Rosenthaler et al., 2014] Although several cannabinoids have been shown to deposit in brain tissue [Alozie, et al., 1980; Deiana et al., 2012], THCA seems to have limited access to the CNS or ability to cross the blood brain barrier (BBB), likely due to its carboxylic acid group. [Moreno-Sanz et al., 2016] This means it also does not elicit any psychoactivity. Preliminary research suggests THCA exhibits antineoplastic, neuroprotective, and anti-inflammatory activities via modulation of COX pathways, TRP cation channels, and other immunomodulatory and cell signaling pathways. CBDA, the acidic precursor to CBD, shares the same enhancing activity of CBD at 5-HT1A receptors, even up to 10 times higher than the activity of CBD, but does not show agonism or antagonism at CB1 receptors. [Bolognini et al., 2013; McPartland et al., 2015] CBDA can also inhibit COX1 and COX2, and, at low concentrations (between 1 and 10 μM), targets GPR55, TRPA1, TRPV1, and TRPM8. [Takeda et al., 2008]
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. The definition of hemp from the Agriculture Improvement Act of 2018 lacked a proper citation in the original; one was made for this version. The original is missing the citation for Fernandez et al. 2020; the correct citation was found and used for this version.