Journal:Accelerated solvent extraction of terpenes in cannabis coupled with various injection techniques for GC-MS analysis
| Full article title | Accelerated solvent extraction of terpenes in cannabis coupled with various injection techniques for GC-MS analysis |
|---|---|
| Journal | Frontiers in Chemistry |
| Author(s) | Myers, Colton; Herrington, Jason S.; Hamrah, Paul; Anderson, Kelsey |
| Author affiliation(s) | Restek Corporation, Verity Analytics |
| Primary contact | colton dot myers at restek dot com |
| Year published | 2021 |
| Volume and issue | 9 |
| Article # | 619770 |
| DOI | 10.3389/fchem.2021.619770 |
| ISSN | 2296-2646 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.frontiersin.org/articles/10.3389/fchem.2021.619770/full |
| Download | https://www.frontiersin.org/articles/10.3389/fchem.2021.619770/pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
The cannabis market is expanding exponentially in the United States. As state-wide legalization efforts increase, so also do demands for analytical testing methodologies. One of the main tests conducted on cannabis products is the analysis for terpenes. This research focused on implementation of accelerated solvent extraction (ASE), utilizing surrogate matrix matching, and evaluation of traditional vs. more modern sample introduction techniques for analyzing terpenes via gas chromatography–mass spectrometry (GC-MS). Introduction techniques included headspace syringe (HS syringe), HS-solid-phase microextraction Arrow (HS-SPME Arrow), direct immersion-SPME Arrow (DI-SPME Arrow), and liquid injection syringe (LI syringe). The LI syringe approach was deemed the most straightforward and robust method, with terpene working ranges of 0.04–5.12 μg/mL; r2 values of 0.988–0.996 (0.993 average); limit of quantitation values of 0.017–0.129 μg/mL (0.047 average); analytical precisions of 2.58–9.64% RSD (1.56 average); overall ASE-LI-syringe-GC-MS method precisions of 1.73–14.6% RSD (4.97 average); and % recoveries of 84.6–98.9% (90.2 average) for the 23 terpenes of interest. Sample workflows and results are discussed, with an evaluation of the advantages/limitations of each approach and opportunities for future work.
Keywords: accelerated solvent extraction (ASE), terpenes, solid-phase microextraction (SPME), solid-phase microextraction Arrow (SPME Arrow), gas chromatography–mass spectrometry (GC-MS)
Introduction
The legal cannabis market is one of the fastest growing markets across the globe. In 2019, cannabis use for medicinal purposes in the United States generated $4 billion to $4.9 billion in sales, compared to the adult-use estimates between $6.6 billion and $8.1 billion.[1] As the United States and additional countries continue to legalize the use of medicinal and recreational cannabis, analytical testing demands increase. A 2020 report by Market Data Forecast valued the global cannabis testing market at $1,218.0 million in 2019 and estimated it to be growing at a compound annual growth rate (CAGR) of 12.42%.[2] The market is projected to almost double at $2,187.3 million by 2024.[2] Of the examinations conducted in cannabis testing laboratories, terpene profiling is a popular analysis, regardless of state regulations.
Terpenes are a naturally occurring set of organic compounds, which are commonly found in plants, and are typically strong in odor.[3] Terpenes are made up of isoprene units and are classified by the number of their isoprene units.[3] The two types of terpenes that are commonly analyzed in the cannabis testing industry are monoterpenes, which have two isoprene units, and sesquiterpenes, which have three isoprene units. Over 100 terpenes have been identified in different cannabis chemical varieties (chemovars).[4] Each cannabis chemovar has its own unique terpene profile, giving consumers different aromas, flavors, and experiences depending on the chemovar they use. According to Russo et al., terpenes play a major role in the entourage effect, which is the synergistic interaction between phytocannabinoids and terpenoids with respect to treating numerous ailments (e.g., depression).[5] The desire to understand and capitalize on this entourage effect is the motivation for terpene testing in the cannabis industry.
Terpenes have been analyzed in numerous commodities within the food and beverage industry. Previous studies have looked at a variety of matrices (e.g., tequila) and have used different analytical techniques (e.g., solid-phase microextraction [SPME]) to conduct the analysis.[4][6][7][8][9][10][11][12][13][14][15][16][17][18][19] However, only a few studies have shown the analysis of terpenes in cannabis and hemp matrices (e.g., flower, gummy), and their robustness for compliance laboratories remains uncertain. Calvi et al., Ternelli et al., Gaggotti et al., and Stenerson et al. did not perform extractions on cannabis and hemp samples; rather, they added the samples directly to a headspace (HS) vial and demonstrated the analysis of terpenes using HS-SPME.[4][10][15][18] Nguyen et al. utilized a pseudo extraction by adding a solvent to dried material, followed by analysis via headspace gas chromatography–mass spectrometry (HS-GC-MS).[17] The five aforementioned studies appear to lack an exhaustive cannabis or hemp extraction, and therefore this calls into question the real-world applicability of these methods. Furthermore, Calvi et al., Ternelli et al., Gaggotti et al., and Stenerson et al. only focused on profiling the terpenes in the cannabis or hemp matrices studied and therefore only presented qualitative and semi-quantitative data.[4][10][15][18]
Bakro et al., Brown et al., Ibrahim et al., and Shapira et al. extracted cannabis flower with ethanol, hexane, ethyl acetate, and methanol, respectively, and provided quantitative results.[11][12][13][14] However, Bakro et al. only looked at hemp and used a nonspecific gas chromatography with flame-ionization detection (GC-FID) approach, which is cumbersome when attempting to differentiate between coeluting terpenes of interest and matrix interferences.[14] Brown et al. did not provide method accuracies for all targeted terpenes and reported less than desirable linearities, which fell below an r2 value of 0.960 for each terpene of interest.[11]
To date, the most promising methods—presented by Ibrahim et al. and Shapira et al.—utilize exhaustive cannabis and hemp extraction approaches, followed by GC-MS and reported desirable quantitative results.[12][13] Ibrahim et al. and Shapira et al. used sample introduction techniques like liquid injection without filtration and static headspace GC-MS (SHS-GC-MS), respectively. More importantly, none of the aforementioned studies accounted for matrix effects, as they all used solvent-based calibrations and, due to the complexity and dirtiness of cannabis matrices, this could lead to inaccurate reporting.[20] In addition, these studies did not evaluate more modern sample extraction approaches [e.g., accelerated solvent extraction (ASE)] or sample introduction techniques (e.g., direct immersion-SPME Arrow [DI-SPME Arrow]).
The following study was conducted to evaluate more modern sample preparation and introduction techniques and demonstrate their potential value to cannabis compliance testing laboratories in need of guidance for qualitative and quantitative terpenes analysis. In addition, this study evaluated accelerated solvent extraction (ASE 350) of terpenes in cannabis samples, which is commonly used in other markets within the analytical testing industry.[21][22][23][24] Furthermore, to avoid potentially inaccurate reporting, matrix-matched standards were used for calibration. Finally, the more traditional headspace syringe (HS syringe) and liquid injection syringe (LI syringe) approaches were compared to the more modern HS-solid-phase microextraction Arrow (HS-SPME Arrow) and DI-SPME Arrow, which have recently demonstrated enhanced robustness and improved sensitivity over traditional SPME fibers.[25]
Experimental
The following experimental sections describe the detailed procedures utilized during the three main parts of this manuscript:
1. HS syringe vs. HS-SPME Arrow vs. DI-SPME Arrow for the determination of the preferred sample introduction approach with the use of terpenes in solution; 2. Terpene extraction evaluation for the evaluation of an ideal terpene extraction method for cannabis flower; and 3. Combining the information gathered from the prior two procedures for a final comparison with an existing validated LI syringe technique (i.e., validated with the California Bureau of Cannabis Control [BCC]), outlined in DI-SPME Arrow vs. LI syringe.
Materials and reagents
Hop pellets were obtained from the Beverage Factory (San Diego, CA, United States) and stored at −10 °C for one hour. Dried cannabis material was obtained from Cream of the Crop (San Diego, CA, United States) and stored at −10 °C for one hour. Cannabis Terpene Standards #1 and #2 (cat# 34095 and 34096) were purchased from Restek Corporation (Bellefonte, PA, United States). Napthalene-d8 was purchased from Restek Corporation (Bellefonte, PA, United States). Isopropanol (IPA) was purchased from Filtrous (LCMS Grade) and 1.1 mm, 120 µm DVB/PDMS SPME Arrows were purchased from Restek Corporation (Bellefonte, PA, United States).
HS syringe vs. HS-SPME Arrow vs. DI-SPME Arrow
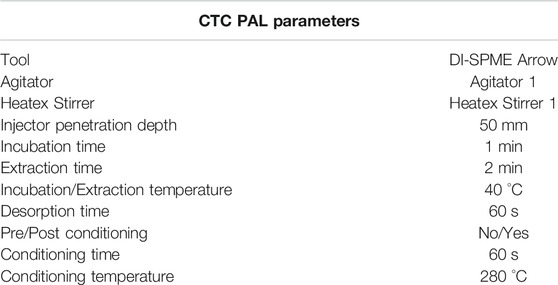
The following experiments were conducted to evaluate the differences between HS syringe, HS-SPME Arrow, and DI-SPME Arrow as sample introduction techniques. Each technique was evaluated using Cannabis Terpene Standard #1 and #2 (cat# 34095 and 34096) from Restek Corporation (Bellefonte, PA, United States). Samples were evaluated using the same GC-MS conditions shown in Supplementary Table S1. For the HS syringe and HS-SPME Arrow samples, a standard stock solution was made by diluting both standards into one solution for a final concentration of 5 μg/mL in IPA. Samples were prepared by adding 1.5 g of NaCl to a 20 mL HS vial, followed by 1 mL of 5 μg/mL stock solution and 4 mL of water for a final concentration of 1 μg/mL (see sampling conditions in Supplementary Tables S2, S3). Previous work (results not shown) demonstrated that HS-SPME Arrow analyte responses were higher and more reproducible when using an incubation temperature of 40 °C or less, hence having lower incubation temperature compared to the HS syringe method. The DI-SPME Arrow samples were prepared by diluting both standards into one stock solution for a final concentration of 20 μg/mL. To a 20 mL HS vial, 1 mL of the 20 μg/mL stock solution was added, followed by 19 mL of water for a final concentration of 1 μg/mL (same concentration for HS syringe and HS-SPME Arrow; see sampling conditions in Supplementary Table S3). Each technique was run in triplicate for the initial evaluation of sample introduction techniques.
Terpene extraction evaluation
An evaluation of the terpene extraction processes was conducted to understand the advantages and limitations of certain techniques. Extractions using the Dionex Accelerated Solvent Extractor (ASE 350) were compared to a hand-solvent extraction for terpene analysis. Three chemical varieties (chemovars) were used to evaluate both extraction techniques. Cannabis flower was frozen at −10 °C for one hour, then homogenized on a sheet pan with a rolling pin. For ASE 350 extractions, 0.5 g of homogenized cannabis flower was weighed and added to a 10 mL stainless steel ASE 350 cell, and the remaining cell volume was lightly packed with diatomaceous earth. The cell was then extracted using the parameters in Table 1. The extract was then diluted to 12 mL in a graduated cylinder due to both convenience and the fact that this approach achieved the desired data quality objectives of this study (e.g., method precision RSDs <15%). However, future researchers may consider the use of volumetric flasks to achieve better precision. One mL of the cannabis flower extract was added to a 2.5 mL autosampler vial and then analyzed. For hand extractions, 0.5 g of homogenized cannabis flower was weighed and added to a 50 mL centrifuge tube, followed by 12 mL of IPA. Samples were vortexed for three minutes and sonicated at 40 °C for five minutes. Samples were then centrifuged in a Sorvall RT7 Plus centrifuge for three minutes. One mL of the supernatant was added to a 2.5 mL autosampler vial and then analyzed. ASE 350 and hand extractions were analyzed via GC-FID.
|
DI-SPME Arrow vs. LI syringe
Results from the experiments outlined in the prior two steps indicated DI-SPME Arrow was the preferred sample introduction approach, and ASE was the ideal terpene extraction technique for cannabis. This information was then utilized for a comparison to an existing validated LI syringe method. However, the experiments conducted in HS syringe vs. HS-SPME Arrow vs. DI-SPME Arrow were carried out in Pennsylvania, and the use of cannabis flower necessitated a fully licensed laboratory, which was located in California. The DI-SPME Arrow parameters, outlined in Table 2, had been further optimized for terpenes analysis in cannabis and used in the California laboratory; however, they are only slightly different from the initial DI-SPME Arrow parameters, as outlined in Supplementary Table S3. In addition, a gas chromatography–tandem mass spectrometry (GC-MS/MS) method was used in single quad MS mode in the California laboratory, since single quad MS is more common for this analysis (see parameters in Table 3). Furthermore, a selected ion monitoring (SIM) method (Supplementary Table S4) was utilized to help eliminate background noise and provide better sensitivity. LI syringe was only evaluated in California using the parameters listed in Table 3.
|
|
Hops pellets and cannabis flower preparation
Hops pellets were utilized as a terpene-free surrogate to matrix match cannabis flower for the following DI-SPME Arrow vs. LI syringe data: calibration curves, laboratory control samples (LCS), continuing calibration verification (CCV) samples, detection limit, and analytical precision samples. Hops were crushed and homogenized on a sheet pan with a rolling pin. The crushed hops were then cleaned with a proprietary solvent cleaning process to eliminate the presence of terpenes. Following solvent cleaning, the hops were dried in an oven. For the DI-SPME Arrow and LI syringe method precision experiments, cannabis shake (small pieces of cannabis flower that break off of larger buds) was homogenized and utilized. For the cannabis chemovar experiments, the flower was crushed and homogenized on a sheet pan using a rolling pin.
Accelerated solvent extractor
The following DI-SPME Arrow vs. LI syringe experiments were conducted utilizing hops pellets and cannabis flowers, which were extracted using an ASE 350 with the parameters previously shown in Table 1. For all of the following DI-SPME Arrow and LI syringe experiments, either 0.5 g of cleaned hops or 0.5 g of cannabis flower was weighed out and placed into a 10 mL ASE 350 stainless steel extraction cell. Diatomaceous earth was then slowly added and lightly packed to fill the remaining volume in the cell. Samples were then extracted using IPA. Other work has shown that extracting with IPA can lead to poor peak shape for the terpenes of interest.[26] However, IPA gave desirable peak shape for this study and was used because of its cost, convenience, and toxicity relative to other solvents demonstrated for cannabis extractions.
After ASE processing
After ASE extraction, all extracts, which were typically between 10 and 11 mL, were brought to a final volume of 12 mL in order to consistently evaluate extracts of the same volume. Using a 3 mL Luer lock syringe with 0.22 µm filter, 3 mL of extract was filtered. For DI-SPME Arrow experiments, 1 mL of the filtered extract was added to 19 mL of LCMS grade water (i.e., 20 mL final volume) in a 20 mL headspace vial. In addition, 20 µL of 100 μg/mL internal standard (ISTD) solution was added. Subsequently, the headspace vial was capped and spun for 10 seconds. For LI syringe experiments, 500 µL of the filtered extract was added to a 2 mL autosampler vial. In addition, 5 µL of the 10 μg/mL ISTD solution was added. Subsequently, the autosampler vial was capped and spun for 10 seconds.
References
- ↑ Stelton-Holtmeier, J. (25 August 2020). "Chart: Nationwide sales of adult-use cannabis further eclipse those of medical marijuana". Marijuana Business Daily. https://mjbizdaily.com/chart-nationwide-sales-of-adult-use-cannabis-further-eclipse-those-of-medical-marijuana/. Retrieved 31 August 2020.
- ↑ 2.0 2.1 "Chart: Nationwide sales of adult-use cannabis further eclipse those of medical marijuana". February 2020. https://www.marketdataforecast.com/market-reports/cannabis-testing-market. Retrieved 31 August 2020.
- ↑ 3.0 3.1 Nuutinen, T. (2018). "Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus". European Journal of Medicinal Chemistry 157: 198–228. doi:10.1016/j.ejmech.2018.07.076. PMID 30096653.
- ↑ 4.0 4.1 4.2 4.3 Calvi, L.; Pentimalli, D.; Panseri, S. et al. (2018). "Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap) approach". Journal of Pharmaceutical and Biomedical Analysis 150: 208–19. doi:10.1016/j.jpba.2017.11.073.
- ↑ Russo, E.B. (2011). "Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects". British Journal of Pharmacology 163 (7): 1344-64. doi:10.1111/j.1476-5381.2011.01238.x. PMC PMC3165946. PMID 21749363. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC3165946.
- ↑ Abilleira, E.; de Renobales, M.; Nájera, A.I. et al. (2010). "An accurate quantitative method for the analysis of terpenes in milk fat by headspace solid-phase microextraction coupled to gas chromatography–mass spectrometry". Food Chemistry 120 (4): 1162-1169. doi:10.1016/j.foodchem.2009.11.050.
- ↑ Kupska, M.; Chmiel, T.; Jędrkiewicz, R. et al. (2014). "Comprehensive two-dimensional gas chromatography for determination of the terpenes profile of blue honeysuckle berries". Food Chemistry 152: 88–93. doi:10.1016/j.foodchem.2013.11.129. PMID 24444910.
- ↑ Cacho, J.I.; Campillo, N.; Viñas, P. et al. (2015). "Evaluation of three headspace sorptive extraction coatings for the determination of volatile terpenes in honey using gas chromatography-mass spectrometry". Journal of Chromatography A 1399: 18–24. doi:10.1016/j.chroma.2015.04.041. PMID 25958092.
- ↑ Jeleń, H.H.; Gracka, A. (2015). "Analysis of black pepper volatiles by solid phase microextraction-gas chromatography: A comparison of terpenes profiles with hydrodistillation". Journal of Chromatography A 1418: 200–209. doi:10.1016/j.chroma.2015.09.065. PMID 26427328.
- ↑ 10.0 10.1 10.2 Stenerson, K.K.; Halpenny, M.R. (2017). "Analysis of Terpenes in Cannabis Using Headspace Solid-Phase Microextraction and GC–MS". LCGC 35 (5): 27–32. https://www.chromatographyonline.com/view/analysis-terpenes-cannabis-using-headspace-solid-phase-microextraction-and-gc-ms.
- ↑ 11.0 11.1 11.2 Brown, A.K.; Xia, Z.; Bulloch, P. et al. (2019). "Validated quantitative cannabis profiling for Canadian regulatory compliance - Cannabinoids, aflatoxins, and terpenes". Analytica Chimica Acta 1088: 79–88. doi:10.1016/j.aca.2019.08.042. PMID 31623719.
- ↑ 12.0 12.1 12.2 Ibrahim, E.A.; Wang, M.; Radwan, M.M. et al. (2019). "Analysis of Terpenes in Cannabis sativa L. Using GC/MS: Method Development, Validation, and Application". Planta Medica 85 (5): 431–38. doi:10.1055/a-0828-8387. PMID 30646402.
- ↑ 13.0 13.1 13.2 Shapira, A.; Berman, P. Futoran, K. et al. (2019). "Tandem Mass Spectrometric Quantification of 93 Terpenoids in Cannabis Using Static Headspace Injections". Analytical Chemistry 91 (17): 11425–32. doi:10.1021/acs.analchem.9b02844. PMID 31369251.
- ↑ 14.0 14.1 14.2 Bakro, F.; Jedryczka, M.; Wielgusz, K. et al. (2020). "Simultaneous determination of terpenes and cannabidiol in hemp (Cannabis sativa L.) by fast gas chromatography with flame ionization detection". Journal of Separation Science 43 (14): 2817–26. doi:10.1002/jssc.201900822.
- ↑ 15.0 15.1 15.2 Gaggiotti, S.; Palmieri, S.; Pelle, F.D. et al. (2020). "Piezoelectric peptide-hpDNA based electronic nose for the detection of terpenes; Evaluation of the aroma profile in different Cannabis sativa L. (hemp) samples". Sensors and Actuators B: Chemical 308: 127697. doi:10.1016/j.snb.2020.127697.
- ↑ {{cite journal |title=Multivariate optimization of headspace solid-phase microextraction coupled to gas chromatography-mass spectrometry for the analysis of terpenoids in sparkling wines |journal=Talanta |author=Muñoz-Redondo, J.M.; Ruiz-Moreno, M.J.; Puertas, B. et al. |volume=208 |at=120483 |year=2020 |doi=110.1016/j.talanta.2019.120483}
- ↑ 17.0 17.1 Nguyen, T.-D.; Riordan-Short, S.; Dang, T.-T.T. et al. (2020). "Quantitation of Select Terpenes/Terpenoids and Nicotine Using Gas Chromatography-Mass Spectrometry with High-Temperature Headspace Sampling". ACS Omega 5 (10): 5565-5573. doi:10.1021/acsomega.0c00384. PMC PMC7081649. PMID 32201850. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=PMC7081649.
- ↑ 18.0 18.1 18.2 Ternelli, M.; Brighenti, V.; Anceschi, L. et al. (2020). "Innovative methods for the preparation of medical Cannabis oils with a high content of both cannabinoids and terpenes". Journal of Pharmaceutical and Biomedical Analysis 186: 113296. doi:10.1016/j.jpba.2020.113296. PMID 32334134.
- ↑ Zhang, X.; Wang, C.; Wang, L. et al. (2020). "Optimization and validation of a head space solid-phase microextraction-arrow gas chromatography-mass spectrometry method using central composite design for determination of aroma compounds in Chinese liquor (Baijiu)". Journal of Chromatography A 1610: 460584. doi:10.1016/j.chroma.2019.460584. PMID 31607446.
- ↑ Raposo, F.; Barceló, D. (2021). "Challenges and strategies of matrix effects using chromatography-mass spectrometry: An overview from research versus regulatory viewpoints". TrAC Trends in Analytical Chemistry 134: 116068. doi:10.1016/j.trac.2020.116068.
- ↑ Ligor, M.; Stankevičius, M.; Wenda-Piesik, A. et al. (2014). "Comparative Gas Chromatographic–Mass Spectrometric Evaluation of Hop (Humulus lupulus L.) Essential Oils and Extracts Obtained Using Different Sample Preparation Methods". Food Analytical Methods 7: 1433–42. doi:10.1007/s12161-013-9767-5.
- ↑ Chiesa, L.M.; Labella, G.F.; Panseri, S. et al. (2017). "Accelerated solvent extraction by using an 'in-line' clean-up approach for multiresidue analysis of pesticides in organic honey". Food Additives & Contaminants: Part A 34 (5): 809–18. doi:10.1080/19440049.2017.1292558. PMID 28277176.
- ↑ Hu, A.; Qiu, M.; Liu, H. et al. (2020). "Simultaneous determination of phthalate diesters and monoesters in soil using accelerated solvent extraction and ultra-performance liquid chromatography coupled with tandem mass spectrometry". Journal of Chromatography A 1626: 104366. doi:10.1016/j.microc.2019.104366.
- ↑ Min, N.; Yao, J.; Amde, M. et al. (2020). "Accelerated solvent extraction combined with GC–MS: A convenient technique for the determination and compound-specific stable isotope analysis of phthalates in mine tailings". Microchemical Journal 153: 461347. doi:10.1016/j.chroma.2020.461347. PMID 32797827.
- ↑ Herrington, J.S.; Gómez-Ríos, G.A.; Myers, C. et al. (2020). "Hunting Molecules in Complex Matrices with SPME Arrows: A Review". Separations 7 (1): 12. doi:10.3390/separations7010012.
- ↑ Krill, C.; Rochfort, S.; Spangenberg, G. (2020). "A High-Throughput Method for the Comprehensive Analysis of Terpenes and Terpenoids in Medicinal Cannabis Biomass". Metabolites 10 (7): 276. doi:10.3390/metabo10070276.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. The original article lists references in alphabetical order; this wiki organizes them by order of appearance, by design.